The 1400 metabolite-mediated relationship between 91 inflammatory cytokines and migraine: An exploratory two-step Mendelian randomization study
Abstract
Background
Inflammatory cytokines and migraines have been associated in previous research, but the underlying mechanisms of action are still elusive. The biological functions of metabolites are crucial in the onset of migraine. Our goals were to clarify the cause-and-effect connection between inflammatory cytokines and migraines and explore the potential mediating function of metabolites.
Methods
Utilizing summary-level data from genome-wide association studies (GWAS), we conducted two-sample Mendelian randomization (MR) analyses to evaluate the possible causal connection between inflammatory cytokines and migraines. A two-step MR analysis was employed to further investigate the potential mediating pathways of metabolites.
Results
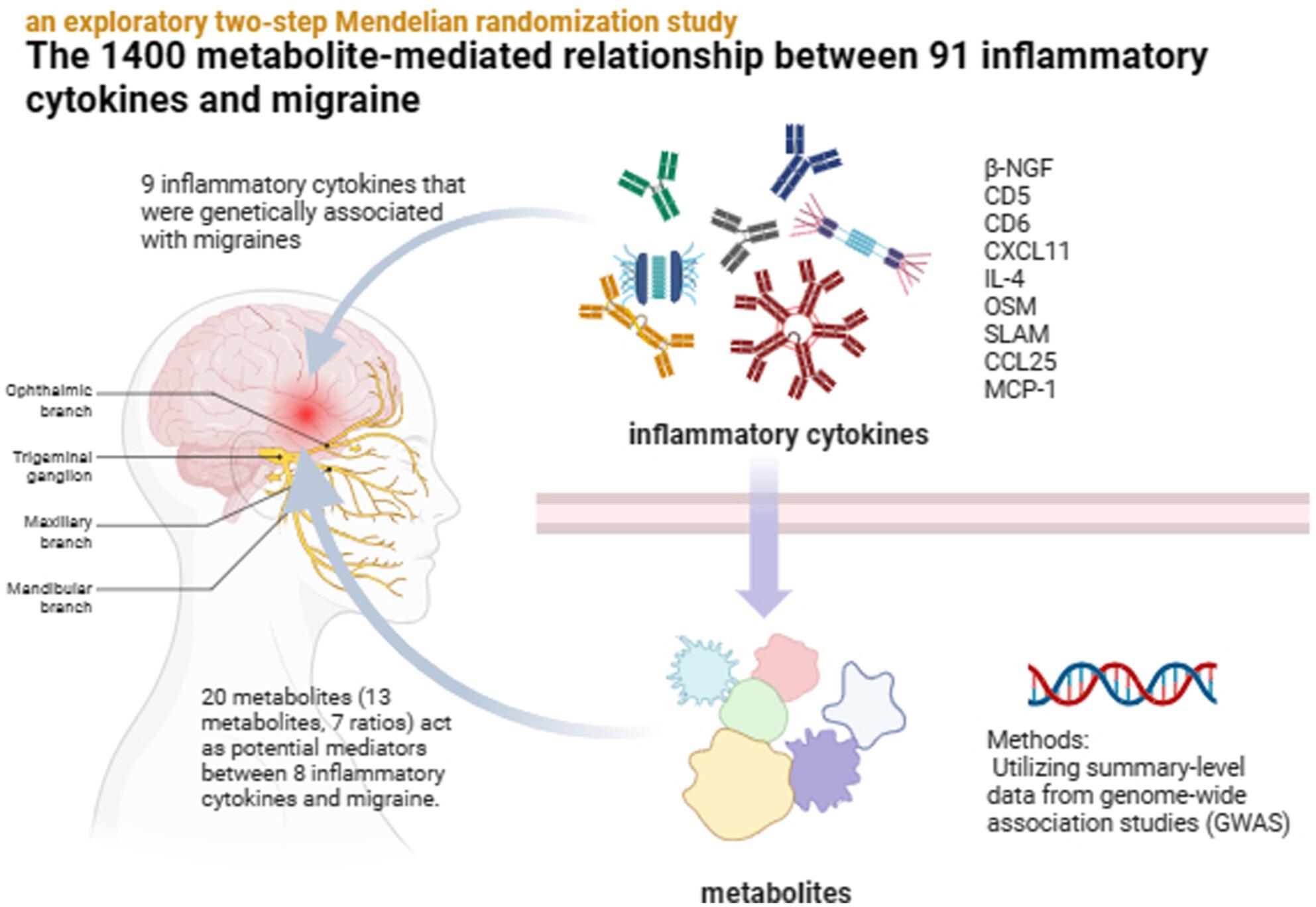
MR analysis identified a total of 9 inflammatory cytokines that were genetically associated with migraines, and we subsequently identified 21 mediated relationships, with 20 metabolites (13 metabolites, 7 ratios) acting as potential mediators between 8 inflammatory cytokines and migraine. The 9 inflammatory cytokines were beta-nerve growth factor levels (β-NGF), T-cell surface glycoprotein CD5 levels (CD5), T-cell surface glycoprotein CD6 isoform levels (CD6), C-X-C motif chemokine 11 levels (CXCL11), interleukin-4 levels (IL-4), oncostatin-M levels (OSM), signalling lymphocytic activation molecule levels (SLAM), C-C motif chemokine 25 levels (CCL25) and monocyte chemoattractant protein-1 levels (MCP-1).
Conclusion
Our research findings provide evidence for both a causal connection between inflammatory cytokines and migraines, as well as a metabolite-mediated pathway. These biomarkers facilitate the detection, diagnosis and treatment of migraines while offering fresh perspectives on their underlying mechanisms.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


