Effects of L-NAME and air exposure on mitochondrial energetic markers, thyroid hormone receptor/regulator system and stress/ease-responsive receptor expression in the brain/gut axis of zebrafish
IF 3.9
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2024-09-20
DOI:10.1016/j.cbpc.2024.110043
引用次数: 0
Abstract
As a signal molecule, nitric oxide (NO) has several physiological actions in fish. However, the action of NO on the brain/gut axis, a classic inter-organal axis that bridges the gastrointestinal tract and the CNS, still requires more understanding. The short-term in vivo action of a NO inhibitor, N-omega-nitro-L-arginine methyl ester (L-NAME), on mitochondrial energetic markers and the receptor expression of thyroid hormone (TH) and neuroendocrine hormones involved in stress/ease response was tested in the brain/gut axis of zebrafish exposed to either in non-stressed or air-exposed condition. L-NAME treatment decreased the NO content in brain and gut segments in non-stressed fish but rose upon L-NAME treatment in air-exposed fish that corresponded with the activation of inos, nnos, hif1a and hif1an transcript expressions. The brain/gut segments that showed spatial and differential sensitivity to L-NAME, modified the transcript expression patterns of stress (adra2da, adrb1, nr3c2)- and ease-responsive (htr2b, slc6a4a, mtnr1aa) hormone receptors. The expression pattern of the TH receptor/regulator system (thra, thrb, dio1, dio2, dio3) becomes more active in gut segments than brain segments upon L-NAME challenge in stressed zebrafish. The data provide evidence for a novel role of NO as an integrator of brain/gut axis segments in zebrafish, where the endogenously produced NO in mid-brain/posterior-gut axis aligns together upon air-exposure stress, providing a lead role to the posterior gut that activates and directs the neuroendocrine receptor expressions of stress/ease responsive genes. The data further invites studies exploring the therapeutic potential of L-NAME in this biomedical model to control the brain/gut axis segments.
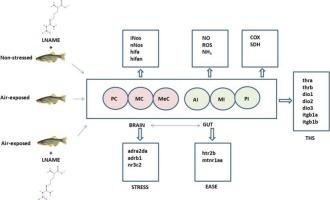
L-NAME和空气暴露对斑马鱼脑/肠轴线粒体能量标记、甲状腺激素受体/调节器系统和应激/酶反应受体表达的影响。
作为一种信号分子,一氧化氮(NO)在鱼类中有多种生理作用。然而,一氧化氮对脑/肠轴(连接胃肠道和中枢神经系统的典型器官间轴)的作用仍有待进一步了解。研究人员测试了 NO 抑制剂 N-欧米伽-硝基-L-精氨酸甲酯(L-NAME)在体内对线粒体能量标记、甲状腺激素(TH)受体表达以及涉及应激/缓解反应的神经内分泌激素的短期作用。L-NAME处理降低了非应激鱼类脑部和肠道中的NO含量,但L-NAME处理后,暴露于空气中的鱼类脑部和肠道中的NO含量升高,这与inos、nnos、hif1a和hif1an转录本表达的激活相对应。对 L-NAME 表现出空间和差异敏感性的脑/肠段改变了应激(adra2da、adrb1、nr3c2)和缓解(htr2b、slc6a4a、mtnr1aa)激素受体转录本的表达模式。在应激斑马鱼受到 L-NAME 挑战时,TH 受体/调节器系统(thra、thrb、dio1、dio2、dio3)的表达模式在肠段比在脑段更活跃。这些数据证明了 NO 在斑马鱼中作为大脑/肠道轴段的整合者所扮演的新角色,在暴露于空气的应激状态下,中脑/后肠道轴段内源性产生的 NO 会聚集在一起,为后肠道提供主导作用,激活并引导应激/缓解反应基因的神经内分泌受体表达。这些数据进一步促进了研究,探索 L-NAME 在这一生物医学模型中控制大脑/肠道轴段的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


