NF-κB p105-mediated nuclear translocation of ERK is required for full activation of IFNγ-induced iNOS expression
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Inducible nitric oxidase (iNOS) encoded by Nos2 is a representative IFNγ-inducible effector molecule that plays an important role in both innate and adaptive immunity. In the present study, we demonstrated that full-length NF-κB p105 (p105), which is a precursor of NF-κB p50 (p50), is required for full activation of IFNγ-induced iNOS expression in the RAW264.7 mouse macrophage cell line. In comparison to wild-type (WT) RAW264.7 cells, p105 KO RAW264.7 (p105 KO) cells completely lost IFNγ-induced iNOS expression. Despite the limited effect of exogenous expression of p50 in p105 KO cells on IFNγ-induced Nos2 promoter activity, p105 expression fully restored IFNγ-induced Nos2 promoter activity to a level comparable to that of WT cells, suggesting an important role for full-length p105 in IFNγ-induced iNOS expression. While the expression and phosphorylation of JAK1 and STAT1 were rather enhanced in p105 KO cells, the phosphorylation of c-Jun downstream of MAPK signaling was decreased. IFNγ-induced phosphorylation of ERK, a kinase for IFNγ-induced c-Jun phosphorylation, was not significantly reduced in p105 KO cells, although the nuclear activity of ERK was significantly decreased due to its reduced translocation to the nucleus. Expression of iNOS, nuclear translocation of ERK, and phosphorylation of c-Jun were restored by stable supplementation of p105 in p105 KO cells. These results suggest that p105 is required for the nuclear translocation of ERK and the subsequent phosphorylation of c-Jun, which are necessary for full activation of IFNγ-induced iNOS expression. Reduced nuclear translocation of ERK in p105 KO cells was also observed in the activation of ERK following serum starvation, further suggesting that the involvement of p105 in ERK nuclear translocation is not limited to IFNγ-stimulated cells but is a more general function of p105.
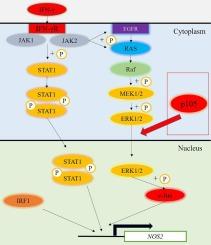
NF-κB p105 介导的 ERK 核转位是全面激活 IFNγ 诱导的 iNOS 表达所必需的。
由 Nos2 编码的诱导型一氧化氮酶(iNOS)是一种具有代表性的 IFNγ 诱导型效应分子,在先天性免疫和适应性免疫中发挥着重要作用。在本研究中,我们证实了全长 NF-κB p105(p105)是 NF-κB p50(p50)的前体,它是完全激活 IFNγ 诱导的 iNOS 在 RAW264.7 小鼠巨噬细胞系中表达所必需的。与野生型(WT)RAW264.7 细胞相比,p105 KO RAW264.7 (p105 KO)细胞完全丧失了 IFNγ 诱导的 iNOS 表达。尽管在 p105 KO 细胞中外源表达 p50 对 IFNγ 诱导的 Nos2 启动子活性的影响有限,但 p105 的表达完全恢复了 IFNγ 诱导的 Nos2 启动子活性,达到了与 WT 细胞相当的水平,这表明全长 p105 在 IFNγ 诱导的 iNOS 表达中起着重要作用。在 p105 KO 细胞中,JAK1 和 STAT1 的表达和磷酸化反而增强了,但 MAPK 信号转导下游的 c-Jun 磷酸化却降低了。IFNγ 诱导的 c-Jun 磷酸化的激酶 ERK 的磷酸化在 p105 KO 细胞中没有明显降低,但 ERK 的核活性因其向细胞核的转位减少而明显降低。在 p105 KO 细胞中稳定补充 p105 后,iNOS 的表达、ERK 的核转位和 c-Jun 的磷酸化均得到恢复。这些结果表明,p105 是 ERK 核转位和 c-Jun 随后磷酸化所必需的,而 ERK 核转位和 c-Jun 磷酸化是全面激活 IFNγ 诱导的 iNOS 表达所必需的。在血清饥饿激活 ERK 的过程中,也观察到 p105 KO 细胞中 ERK 的核转位减少,这进一步表明 p105 参与 ERK 核转位并不局限于 IFNγ 刺激的细胞,而是 p105 的一种更普遍的功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


