Serum exosomal miRNA promote glioma progression by targeting SOS1 via abscopal effect of radiation
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Introduction
Local exposure to ionizing radiation (IR) can induce changes in biological processes in distant tissues and organs. Exosomes are nanoscale vesicles that transport biomolecules, mediate communication between cells and tissues, and can affect the abscopal effects of radiotherapy.
Methods
Mice were treated with 8.0 Gy doses of chest and abdomen IR, after which serum samples were taken 24 h after exposure. Their serum exosomes were then isolated via ultracentrifugation and the small RNA portions were extracted for sequencing and bioinformatic analysis. Exosomes were injected intravenously into the mice to assess their ability to cross the blood-brain barrier (BBB). Glioma cells and glioma stem cells (GSCs) were examined for malignant biological behaviors, stemness, and tumorigenic capacity after co-culturing with different groups of exosomes.
Results
We found that serum exosomes crossed the BBB in mice after local IR exposure-which induced decreases in the expression of BBB tight-junction proteins and increased brain endothelial cell apoptosis. Exosomes from the exposed groups promoted malignant biological behaviors, stemness, and tumorigenic capacity in glioma cells and GSCs by upregulating the expression of SOS1. Phospho-MEK1/2 and Phospho-ERK1/2, of the MAPK signaling pathway, were found to be up-regulated in cells that were co-cultured with the exposing groups of the exosomes. Further analyses demonstrated that differentially expressed levels of miR-93–5p in mouse serum exosomes regulated the cellular expression of SOS1.
Conclusion
Following local IR exposure, serum exosomes cross the BBB to promote the progression of distant gliomas. Exosomal microRNAs play an important role in this process.
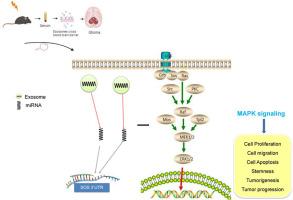
血清外泌体miRNA通过辐射的脱灶效应靶向SOS1,促进胶质瘤的进展。
导言:局部暴露于电离辐射(IR)可诱导远处组织和器官的生物过程发生变化。外泌体是一种纳米级囊泡,可运输生物大分子,介导细胞和组织之间的交流,并可影响放疗的腹腔效应:方法:对小鼠进行8.0 Gy剂量的胸部和腹部红外线照射,然后在照射24小时后采集血清样本。方法:对小鼠进行 8.0 Gy 剂量的胸部和腹部红外照射,照射 24 小时后采集血清样本,然后通过超速离心法分离血清外泌体,提取小 RNA 部分用于测序和生物信息学分析。小鼠静脉注射外泌体,以评估它们穿越血脑屏障(BBB)的能力。在与不同类型的外泌体共培养后,对胶质瘤细胞和胶质干细胞(GSCs)的恶性生物学行为、干性和致瘤能力进行了检测:结果:我们发现,血清外泌体在局部红外暴露后穿过小鼠的BBB,从而诱导BBB紧密连接蛋白表达的减少和脑内皮细胞凋亡的增加。暴露组的外泌体通过上调SOS1的表达,促进了胶质瘤细胞和GSCs的恶性生物学行为、干性和致瘤能力。在与外泌体暴露组共同培养的细胞中,发现MAPK信号通路中的磷酸-MEK1/2和磷酸-ERK1/2被上调。进一步的分析表明,小鼠血清外泌体中表达水平不同的miR-93-5p调节了SOS1的细胞表达:结论:局部红外暴露后,血清外泌体穿过BBB,促进远处胶质瘤的进展。外泌体microRNA在这一过程中发挥着重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
文献相关原料
公司名称
产品信息
阿拉丁
2 % Evans blue
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


