Discovering potential asthma therapeutics targeting hematopoietic prostaglandin D2 synthase: An integrated computational approach
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Allergic asthma, a chronic inflammatory illness that affects millions worldwide, has serious economic and health consequences. Despite advances in therapy, contemporary treatments have poor efficacy and negative effects. This study investigates hematopoietic prostaglandin D2 synthase (HPGDS) as a potential target for novel asthma therapies. Targeting HPGDS may provide innovative treatment methods. A library of phytochemicals was used to find putative HPGDS inhibitors by structure-based and ligand-based virtual screening. Among the 2295 compounds screened, four compounds (ZINC208828240, ZINC95627530, ZINC14727536, and ZINC14711790) demonstrated strong binding affinities of −10.4, −10.3, −9.2, −9.1 kcal/mol respectively with key residues, suggesting their potential as a highly effective HPGDS inhibitor. Molecular dynamics (MD) simulations and Molecular Mechanics Poisson-Boltzmann Surface Area (MMPBSA) computations were further performed to evaluate the stability and binding affinity of the complexes. MD simulations and MMPBSA confirmed that compound ZINC14711790 showed high stability and binding affinity (binding energy −31.52 kcal/mol) than other compounds, including HQL-79, suggesting that this compound might be used as promising inhibitors to treat asthma. RMSD and RMSF analysis also revealed that ZINC14711790 exhibited strong dynamic stability. The findings of this study show the efficacy of ZINC14711790 as HPGDS inhibitors with high binding affinity, dynamic stability, and appropriate ADMET profile.
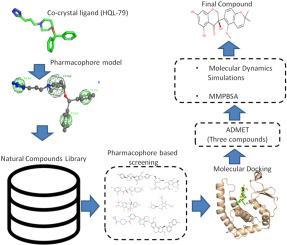
发现针对造血前列腺素 D2 合成酶的潜在哮喘疗法:综合计算方法。
过敏性哮喘是一种影响全球数百万人的慢性炎症性疾病,对经济和健康造成严重后果。尽管治疗手段在不断进步,但当代疗法的疗效不佳且存在负面影响。本研究将造血前列腺素 D2 合成酶(HPGDS)作为新型哮喘疗法的潜在靶点。以 HPGDS 为靶点可提供创新的治疗方法。通过基于结构和配体的虚拟筛选,我们利用植物化学物质文库找到了潜在的 HPGDS 抑制剂。在筛选出的 2295 个化合物中,4 个化合物(ZINC208828240、ZINC95627530、ZINC14727536 和 ZINC14711790)与关键残基的结合亲和力分别为 -10.4、-10.3、-9.2、-9.1 kcal/mol,表明它们具有作为高效 HPGDS 抑制剂的潜力。研究人员进一步进行了分子动力学(MD)模拟和分子力学泊松-波尔兹曼表面积(MMPBSA)计算,以评估复合物的稳定性和结合亲和力。MD 模拟和 MMPBSA 证实,与包括 HQL-79 在内的其他化合物相比,ZINC14711790 复合物表现出较高的稳定性和结合亲和力(结合能 -31.52 kcal/mol),这表明该化合物有望用作治疗哮喘的抑制剂。RMSD 和 RMSF 分析还显示 ZINC14711790 具有很强的动态稳定性。这项研究结果表明,ZINC14711790 作为 HPGDS 抑制剂具有高结合亲和力、动态稳定性和适当的 ADMET 特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


