KA-mediated excitotoxicity induces neuronal ferroptosis through activation of ferritinophagy
Abstract
Objectives
This study aims to elucidate the role of Fe2+ overload in kainic acid (KA)-induced excitotoxicity, investigate the involvement of ferritinophagy selective cargo receptor NCOA4 in the pathogenesis of excitotoxicity.
Methods
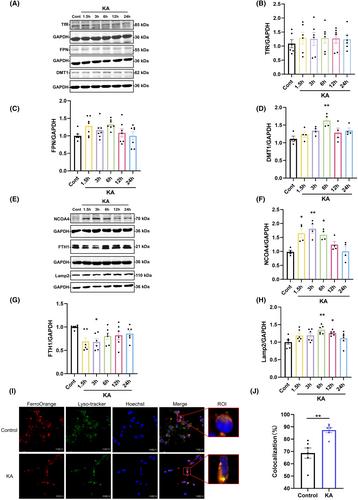
Western blotting was used to detect the expression of FTH1, NCOA4, Lamp2, TfR, FPN, and DMT1 after KA stereotaxic injection into the unilateral striatum of mice. Colocalization of Fe2+ with lysosomes in KA-treated primary cortical neurons was observed by using confocal microscopy. Desferrioxamine (DFO) was added to chelate free iron, a CCK8 kit was used to measure cell viability, and the Fe2+ levels were detected by FerroOrange. BODIPY C11 was used to determine intracellular lipid reactive oxygen species (ROS) levels, and the mRNA levels of PTGS2, a biomarker of ferroptosis, were measured by fluorescent quantitative PCR. 3-Methyladenine (3-MA) was employed to inhibit KA-induced activation of autophagy, and changes in ferritinophagy-related protein expression and the indicated biomarkers of ferroptosis were detected. Endogenous NCOA4 was knocked down by lentivirus transfection, and cell viability and intracellular Fe2+ levels were observed after KA treatment.
Results
Western blot results showed that the expression of NCOA4, DMT1, and Lamp2 was significantly upregulated, while FTH1 was downregulated, but there were no significant changes in TfR and FPN. The fluorescence results indicated that KA enhanced the colocalization of free Fe2+ with lysosomes in neurons. DFO intervention could effectively rescue cell damage, reduce intracellular lipid peroxidation, and decrease the increased transcript levels of PTGS2 caused by KA. Pretreatment with 3-MA effectively reversed KA-induced ferritinophagy and ferroptosis. Endogenous interference with NCOA4 significantly improved cell viability and reduced intracellular free Fe2+ levels in KA-treated cells.
Conclusion
KA-induced excitotoxicity activates ferritinophagy, and targeting ferritinophagy effectively inhibits downstream ferroptosis. Interference with NCOA4 effectively attenuates KA-induced neuronal damage. This study provides a potential therapeutic target for excitotoxicity related disease conditions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


