Iridium-catalyzed reduction of o-hydroxyl phenyl enaminones for the synthesis of propiophenones and their application in 3-methyl chromone synthesis†
IF 4.6
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
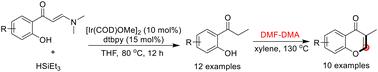
A method of reducing o-hydroxyphenyl enaminones with silane as the reductant to provide o-hydroxyl propiophenones has been achieved with iridium catalysis. The reduction reactions were found to proceed via the assistance of the hydroxyl group in the phenyl ring. In addition, the o-hydroxyl propiophenone products were used for the easy synthesis of 3-methyl chromones by directly incorporating N,N-dimethyl formamide dimethyl acetal (DMF-DMA) without using any catalyst.
铱催化邻羟基苯基烯酮还原合成苯丙酮及其在 3-甲基铬酮合成中的应用。
一种以硅烷为还原剂,通过铱催化还原邻羟基苯丙酮的方法已经实现。研究发现,还原反应是通过苯环上的羟基辅助进行的。此外,邻羟基苯丙酮产品还被用于直接加入 N,N-二甲基甲酰胺二甲基缩醛(DMF-DMA),在不使用任何催化剂的情况下轻松合成 3-甲基色酮。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


