Design strategy and preliminary antiproliferative investigation of a modified lignan skeleton derived from aryne and statin†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The biological activities of natural products (NPs) provided precious resources for the development of new drugs. Numerous studies have shown that statins exhibit cytotoxic potential, which is now an extensive focus of investigation. Herein, a remarkably efficient method for modification of statins using hexadehydro-Diels–Alder (HDDA) arynes has been described. Notably, lactone, as the biologically active group of statins, was removed during the reaction and a novel modified lignan skeleton was generated via the Alder–ene process. Unexpectedly, these statin-derived novel chemical scaffolds exhibited moderate inhibition effects on the proliferation of cancer cells as determined by CCK-8 assays, and the IC50 values were in the micromolar range.
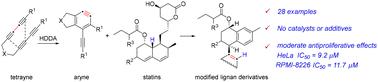
从芳香烃和他汀中提取的改良木质素骨架的设计策略和初步抗增殖研究。
天然产物(NPs)的生物活性为新药开发提供了宝贵的资源。大量研究表明,他汀类药物具有潜在的细胞毒性,这也是目前研究的一个重点。本文介绍了一种利用六去氢-狄尔斯-阿尔德(HDDA)芳香烃修饰他汀类药物的高效方法。值得注意的是,作为他汀类药物生物活性基团的内酯在反应过程中被去除,并通过 Alder-ene 过程生成了一种新型改性木质素骨架。意想不到的是,根据 CCK-8 试验测定,这些他汀类药物衍生的新型化学支架对癌细胞的增殖具有适度的抑制作用,IC50 值在微摩尔范围内。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


