Copper-catalyzed tandem cyclization reaction of ethynylbenzoxazinones and thiols: facile construction of 2-thiomethylene indoles†
IF 4.6
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
The first successful copper-catalyzed decarboxylative cyclization reaction of ethynylbenzoxazinones and thiols has been developed. A rarely studied α-addition process to a copper–allenylidene intermediate promoted this reaction. Using this protocol, a range of 2-thiomethylene indole compounds have been obtained. This methodology offers significant advantages including mild reaction conditions, cheap catalysts, good yields and broad substrate compatibility.
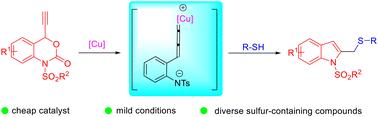
铜催化的乙炔基苯并恶嗪酮与硫醇的串联环化反应:2-硫代亚甲基吲哚的简易构建。
首次成功开发了铜催化的乙炔基苯并恶嗪酮与硫醇的脱羧环化反应。一个很少被研究的亚烯铜中间体α-加成过程促进了这一反应。利用这一方案,获得了一系列 2-硫亚甲基吲哚化合物。该方法具有反应条件温和、催化剂便宜、产率高和底物相容性广等显著优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


