Amide-derived enols in enol–Ugi reactions: expanding horizons for peptidomimetic scaffold synthesis†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A highly efficient enol–Ugi reaction of β,β-diketoamides has been developed using a novel non-heterocyclic amide-stabilised enol. This approach enables a broad reaction scope, affording β-enaminoamide peptidomimetics with constrained conformations due to CH–π interaction and C(sp3)H⋯O hydrogen bonding. Notably, the use of a five-membered cyclic enol is crucial for achieving stable products in excellent yields. This work highlights the potential of the enol–Ugi reaction for constructing diverse peptidomimetic scaffolds.
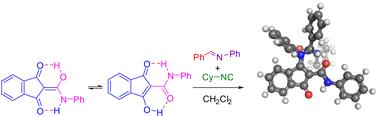
烯醇-乌基反应中的酰胺衍生烯醇:拓宽拟肽支架合成的视野。
利用一种新型的非杂环酰胺稳定烯醇,开发出了β,β-二酮酰胺的高效烯醇-Ugi 反应。这种方法的反应范围很广,由于 CH-π 相互作用和 C(sp3)H⋯O 氢键作用,β-烯酰胺拟肽物的构象受限。值得注意的是,使用五元环烯醇对获得产率优异的稳定产品至关重要。这项工作凸显了烯醇-Ugi 反应在构建多种仿肽支架方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


