Functional profiling of serine, threonine and tyrosine sites
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Systematic perturbation of amino acids at endogenous loci provides diverse insights into protein function. Here, we performed a genome-wide screen to globally assess the cell fitness dependency of serine, threonine and tyrosine residues. Using an adenine base editor, we designed a whole-genome library comprising 817,089 single guide RNAs to perturb 584,337 S, T and Y sites. We identified 3,467 functional substitutions affecting cell fitness and 677 of them involving phosphorylation, including numerous phosphorylation-mediated gain-of-function substitutions that regulate phosphorylation levels of itself or downstream factors. Furthermore, our findings highlight that specific substitution types, notably serine to proline, are crucial for maintaining domain structure broadly. Lastly, we demonstrate that 309 enriched hits capable of initiating cell overproliferation might be potential cancer driver mutations. This study represents an extensive functional profiling of S, T and Y residues and provides insights into the distinctive roles of these amino acids in biological mechanisms and tumor progression. The use of an adenine base editor enables identification of functional serine, threonine and tyrosine residues that impact cell fitness on a genome-wide scale with possible involvement in phosphorylation, structural maintenance and cancer biology.

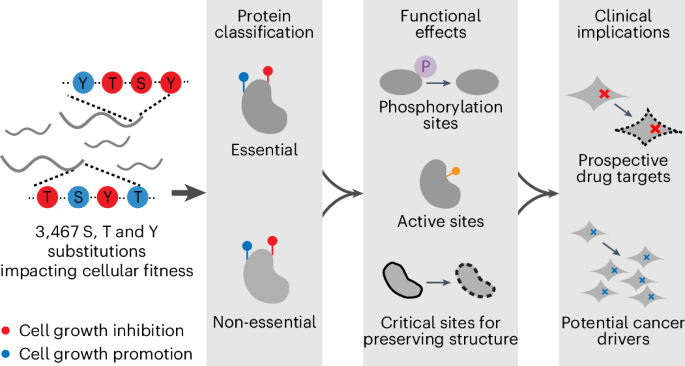
丝氨酸、苏氨酸和酪氨酸位点的功能分析
对内源性位点上的氨基酸进行系统性扰动可提供对蛋白质功能的不同见解。在这里,我们进行了一次全基因组筛选,以全面评估丝氨酸、苏氨酸和酪氨酸残基对细胞适应性的依赖性。利用腺嘌呤碱基编辑器,我们设计了一个由 817,089 个单导 RNA 组成的全基因组文库,以扰乱 584,337 个 S、T 和 Y 位点。我们发现了 3467 个影响细胞活力的功能性置换,其中 677 个涉及磷酸化,包括许多磷酸化介导的功能增益置换,这些置换可调节自身或下游因子的磷酸化水平。此外,我们的研究结果还突出表明,特定的取代类型,尤其是丝氨酸到脯氨酸的取代,对于维持广泛的结构域结构至关重要。最后,我们证明了 309 个能够引发细胞过度增殖的富集突变可能是潜在的癌症驱动突变。这项研究对 S、T 和 Y 残基进行了广泛的功能分析,并深入揭示了这些氨基酸在生物机制和肿瘤进展中的独特作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


