IL-22 resolves MASLD via enterocyte STAT3 restoration of diet-perturbed intestinal homeostasis
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The exponential rise in metabolic dysfunction-associated steatotic liver disease (MASLD) parallels the ever-increasing consumption of energy-dense diets, underscoring the need for effective MASLD-resolving drugs. MASLD pathogenesis is linked to obesity, diabetes, “gut-liver axis” alterations, and defective interleukin-22 (IL-22) signaling. Although barrier-protective IL-22 blunts diet-induced metabolic alterations, inhibits lipid intake, and reverses microbial dysbiosis, obesogenic diets rapidly suppress its production by small intestine-localized innate lymphocytes. This results in STAT3 inhibition in intestinal epithelial cells (IECs) and expansion of the absorptive enterocyte compartment. These MASLD-sustaining aberrations were reversed by administration of recombinant IL-22, which resolved hepatosteatosis, inflammation, fibrosis, and insulin resistance. Exogenous IL-22 exerted its therapeutic effects through its IEC receptor, rather than hepatocytes, activating STAT3 and inhibiting WNT-β-catenin signaling to shrink the absorptive enterocyte compartment. By reversing diet-reinforced macronutrient absorption, the main source of liver lipids, IL-22 signaling restoration represents a potentially effective interception of dietary obesity and MASLD.
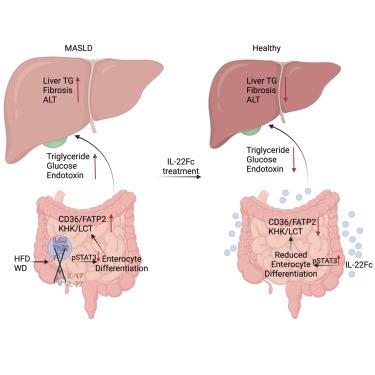
IL-22 通过肠细胞 STAT3 恢复受饮食干扰的肠道稳态来解决 MASLD 问题
代谢功能障碍相关性脂肪性肝病(MASLD)呈指数级增长,与此同时,高能量饮食的消费量也在不断增加,这凸显了对有效的代谢功能障碍相关性脂肪性肝病治疗药物的需求。MASLD的发病机制与肥胖、糖尿病、"肠肝轴 "改变和白细胞介素-22(IL-22)信号传导缺陷有关。虽然具有屏障保护作用的 IL-22 能减弱饮食引起的代谢改变、抑制脂质摄入并逆转微生物菌群失调,但肥胖饮食会迅速抑制小肠定位的先天性淋巴细胞产生这种物质。这导致肠上皮细胞(IECs)中的 STAT3 受抑制,吸收性肠细胞群扩大。通过服用重组 IL-22 逆转了这些维持 MASLD 的畸变,解决了肝软化症、炎症、纤维化和胰岛素抵抗。外源性IL-22通过其IEC受体而非肝细胞发挥治疗作用,激活STAT3并抑制WNT-β-catenin信号传导,从而缩小吸收性肠细胞区。IL-22是肝脏脂质的主要来源,通过逆转饮食强化的高营养素吸收,IL-22信号的恢复代表了一种潜在的有效阻断饮食性肥胖和MASLD的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


