Controlled synthesis and pH-sensitive complexation of poly(methacrylic acid) polyampholytes†
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
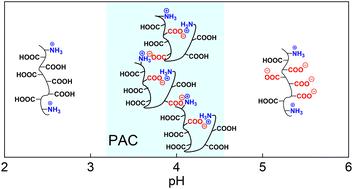
Studies of intra- and intermolecular interactions in pH-responsive polyampholyte solutions are essential for understanding protein molecule solution behavior and cell organelle organization. Understanding and controlling the formation of intra/intermolecular complexes of synthetic polyampholytes can broaden their applications in industry and the biomedical field. Studies of poly(cation-co-anion) statistical copolymer solutions with a predominant content of the same-charge groups in the polymer chain are present in the theory when the factual experimental data are underrepresented. Herein, we explored the controlled synthesis of poly(methacrylic acid) copolymers containing primary amine groups and studied the copolymer solution aggregation under various pH and salt conditions. Well-defined poly(methacrylic acid-co-3-(aminopropyl)-methacryl amide) copolymers (Mw of 45 kDa and 80 kDa, Đ < 1.36) with a varied content of the amine group (PMAA–NH2 from 2 to 6 mol%) were synthesized via reversible addition–fragmentation chain transfer (RAFT) copolymerization. Both computational and experimental studies proved the copolymerization of tert-butyl methacrylate with N-(tert-butoxycarbonyl-aminopropyl)methacrylamide where the second monomer is less active in copolymerization due to strong interaction with a chain-transfer agent (CTA). We found that the resulting PMAA–NH2 copolymers with more than 4 mol% of amine groups form polyampholyte complexes (PACs) in solution in the pH range from 3.1 to 4.8 due to charge compensation. Given the ability of this PMAA–NH2 to undergo multilayer assembly at surfaces and controlled crosslinking, our findings can be further expanded to develop advanced and tunable PMAA thin multilayer hydrogels. The synthesis of PMAA–NH2 copolymers via controlled copolymerization can also lead to facile alternatives for PAC synthesis without using cell-toxic cationic polyelectrolytes such as polyvinylpyridines or polyamines. The copolymers can help develop synthetic routes to novel copolymers and new hydrogel materials with controlled nanostructured architectures, environmentally adaptable microcontinents, PAC-based saloplastics, absorbents, anisotropically structured nanocoatings, and biomedical coatings.
聚(甲基丙烯酸)聚酰胺的可控合成和 pH 值敏感络合
研究 pH 响应型多聚溶液中的分子内和分子间相互作用对于了解蛋白质分子溶液行为和细胞器组织至关重要。了解和控制合成聚阴离子分子内/分子间复合物的形成,可拓宽其在工业和生物医学领域的应用。对聚合物链中同电荷基团含量占主导地位的聚(阳离子-阴离子)统计共聚物溶液的研究,在理论上存在事实实验数据代表性不足的问题。在此,我们探索了含有伯胺基团的聚甲基丙烯酸共聚物的可控合成,并研究了共聚物溶液在不同 pH 值和盐分条件下的聚集情况。通过后续的可逆加成-断裂链转移(RAFT)共聚,我们合成了不同胺基(PMAA-NH2,2-6 mol.%)含量的定义明确的聚(甲基丙烯酸-3-(氨基丙基)-甲基丙烯酸酰胺)共聚物(分子量分别为 45 kDa 和 80 kDa,Đ < 1.36)。计算和实验研究都证明了甲基丙烯酸叔丁酯与 N-(叔丁氧羰基-氨基丙基)甲基丙烯酰胺的共聚,其中第二单体由于与链转移剂(CTA)的强烈相互作用,在共聚中的活性较低。我们发现,在 pH 值为 3.1 至 4.8 的溶液中,由于电荷补偿作用,胺基团含量超过 4 mol.% 的 PMAA-NH2 共聚物会形成聚两性复合物 (PAC)。鉴于这种 PMAA-NH2 能够在表面上进行多层组装和可控交联,我们的研究结果可进一步扩展到开发先进的可调 PMAA 薄多层水凝胶。通过受控共聚合成 PMAA-NH2 共聚物还能为 PAC 合成提供简便的替代方法,而无需使用聚乙烯吡啶或聚胺等对细胞有毒的阳离子聚电解质。这种共聚物有助于开发新型共聚物和新型水凝胶材料的合成路线,这些材料具有可控的纳米结构、环境适应性微大陆、基于 PAC 的盐基塑料、吸收剂、各向异性结构纳米涂层和生物医学涂层。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


