Tuning collective anion motion enables superionic conductivity in solid-state halide electrolytes
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
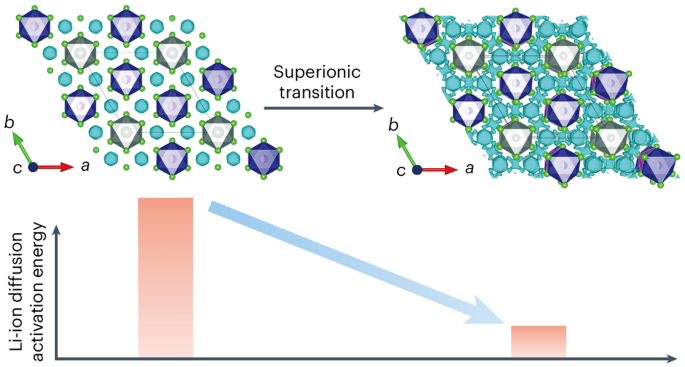
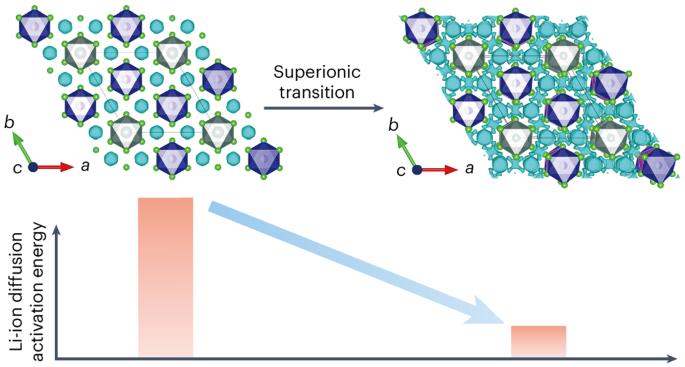
Halides of the family Li3MX6 (M = Y, In, Sc and so on, X = halogen) are emerging solid electrolyte materials for all-solid-state Li-ion batteries. They show greater chemical stability and wider electrochemical stability windows than existing sulfide solid electrolytes, but have lower room-temperature ionic conductivities. Here we report the discovery that the superionic transition in Li3YCl6 is triggered by the collective motion of anions, as evidenced by synchrotron X-ray and neutron scattering characterizations and ab initio molecular dynamics simulations. Based on this finding, we used a rational design strategy to lower the transition temperature and thus improve the room-temperature ionic conductivity of this family of compounds. We accordingly synthesized Li3YClxBr6−x and Li3GdCl3Br3 and achieved very high room-temperature conductivities of 6.1 and 11 mS cm−1 for Li3YCl4.5Br1.5 and Li3GdCl3Br3, respectively. These findings open new routes to the design of room-temperature superionic conductors for high-performance solid batteries. While solid-state lithium-ion batteries offer promising energy densities for safe energy storage, typical solid electrolytes show poor room-temperature ionic conduction. Now the origin of the superionic transition observed in Li3YCl6-type Li-ion conductors is revealed by in-depth crystal structure characterizations and improved ionic conductivities achieved by lowering the transition temperature.
调整阴离子的集体运动实现固态卤化物电解质的超离子导电性
Li3MX6 族卤化物(M = Y、In、Sc 等,X = 卤素)是新兴的全固态锂离子电池固态电解质材料。与现有的硫化物固态电解质相比,它们具有更高的化学稳定性和更宽的电化学稳定性窗口,但室温离子电导率较低。同步辐射 X 射线和中子散射表征以及 ab initio 分子动力学模拟证明,Li3YCl6 中的超离子转变是由阴离子的集体运动触发的。基于这一发现,我们采用了合理的设计策略来降低转变温度,从而提高该系列化合物的室温离子电导率。因此,我们合成了 Li3YClxBr6-x 和 Li3GdCl3Br3,并使 Li3YCl4.5Br1.5 和 Li3GdCl3Br3 的室温电导率分别达到了 6.1 和 11 mS cm-1。这些发现为设计用于高性能固体电池的室温超离子导体开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


