High-throughput identification of repurposable neuroactive drugs with potent anti-glioblastoma activity
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
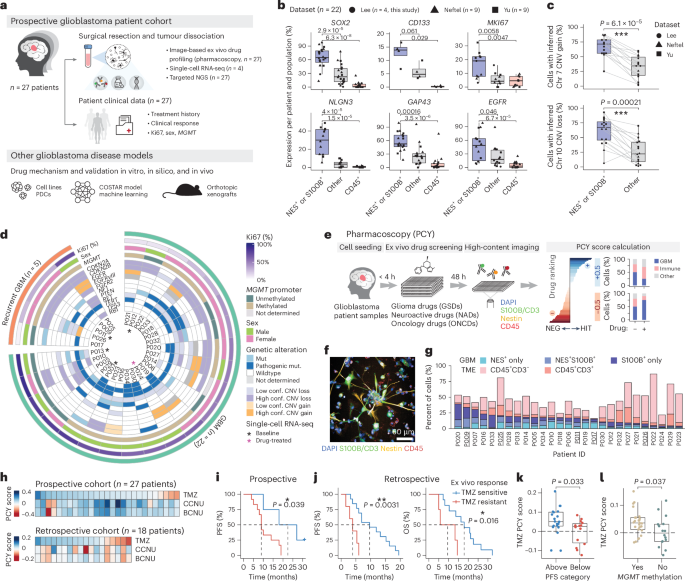
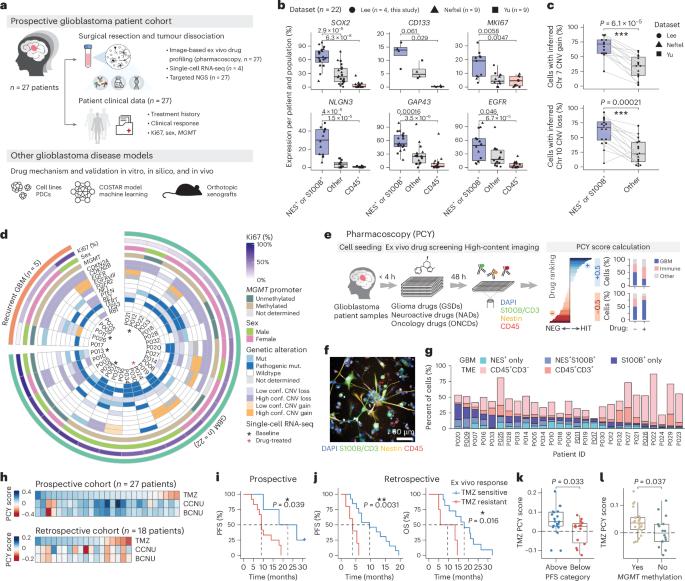
Glioblastoma, the most aggressive primary brain cancer, has a dismal prognosis, yet systemic treatment is limited to DNA-alkylating chemotherapies. New therapeutic strategies may emerge from exploring neurodevelopmental and neurophysiological vulnerabilities of glioblastoma. To this end, we systematically screened repurposable neuroactive drugs in glioblastoma patient surgery material using a clinically concordant and single-cell resolved platform. Profiling more than 2,500 ex vivo drug responses across 27 patients and 132 drugs identified class-diverse neuroactive drugs with potent anti-glioblastoma efficacy that were validated across model systems. Interpretable molecular machine learning of drug–target networks revealed neuroactive convergence on AP-1/BTG-driven glioblastoma suppression, enabling expanded in silico screening of more than 1 million compounds with high patient validation accuracy. Deep multimodal profiling confirmed Ca2+-driven AP-1/BTG-pathway induction as a neuro-oncological glioblastoma vulnerability, epitomized by the anti-depressant vortioxetine synergizing with current standard-of-care chemotherapies in vivo. These findings establish an actionable framework for glioblastoma treatment rooted in its neural etiology. A single-cell ex vivo screening of repurposable drugs in glioblastoma and machine learning of drug–target networks show that anti-tumor neuroactive drugs converge on the AP-1/BTG pathway, based on which prediction models and experimental in vivo and in silico validation identify the anti-depressant vortioxetine as a potential therapeutic agent.
高通量鉴定具有强效抗胶质母细胞瘤活性的可再利用神经活性药物
胶质母细胞瘤是侵袭性最强的原发性脑癌,预后很差,但全身治疗仅限于DNA烷基化化疗。探索胶质母细胞瘤在神经发育和神经生理方面的弱点可能会带来新的治疗策略。为此,我们利用临床一致的单细胞解析平台,在胶质母细胞瘤患者手术材料中系统地筛选了可再利用的神经活性药物。我们对 27 名患者和 132 种药物的 2500 多例体内外药物反应进行了分析,确定了具有强效抗胶质母细胞瘤疗效的各类神经活性药物,并在各种模型系统中进行了验证。对药物靶点网络进行可解释的分子机器学习发现,神经活性趋同于AP-1/BTG驱动的胶质母细胞瘤抑制,从而能够以较高的患者验证准确性扩大对100多万种化合物的硅学筛选。深度多模态剖析证实,Ca2+驱动的AP-1/BTG通路诱导是神经肿瘤胶质母细胞瘤的一个弱点,抗抑郁药伏替西汀在体内与目前的标准化疗药物协同作用就是一个缩影。这些发现为从神经病因出发治疗胶质母细胞瘤建立了一个可行的框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


