Subset-specific mitochondrial stress and DNA damage shape T cell responses to fever and inflammation
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
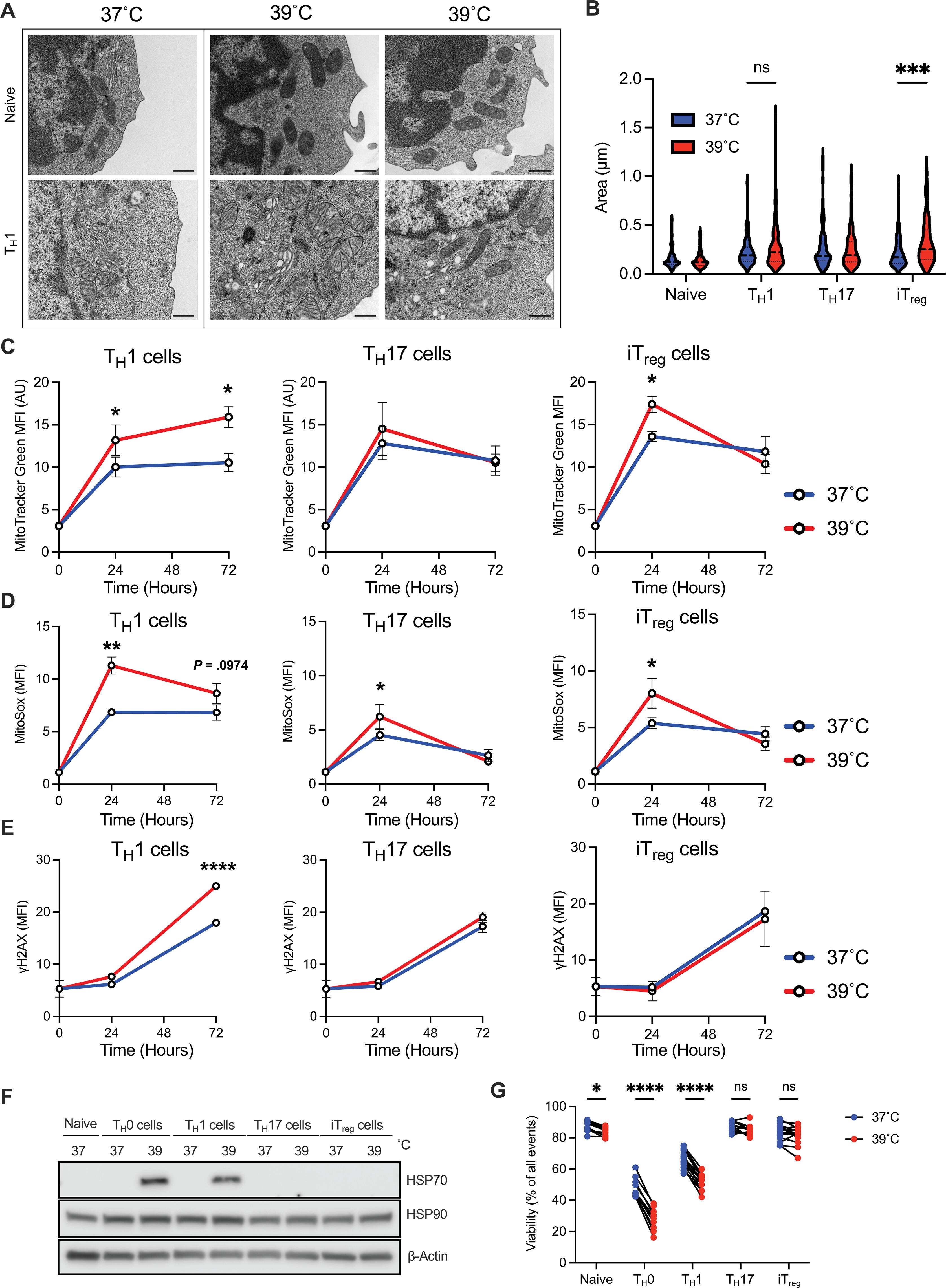
Heat is a cardinal feature of inflammation, yet its impacts on immune cells remain uncertain. We show that moderate-grade fever temperatures (39°C) increased murine CD4 T cell metabolism, proliferation, and inflammatory effector activity while decreasing regulatory T cell suppressive capacity. However, heat-exposed T helper 1 (TH1) cells selectively developed mitochondrial stress and DNA damage that activated Trp53 and stimulator of interferon genes pathways. Although many TH1 cells subjected to such temperatures died, surviving TH1 cells exhibited increased mitochondrial mass and enhanced activity. Electron transport chain complex 1 (ETC1) was rapidly impaired under fever-range temperatures, a phenomenon that was specifically detrimental to TH1 cells. TH1 cells with elevated DNA damage and ETC1 signatures were also detected in human chronic inflammation. Thus, fever-relevant temperatures disrupt ETC1 to selectively drive apoptosis or adaptation of TH1 cells to maintain genomic integrity and enhance effector functions.
亚群特异性线粒体应激和 DNA 损伤决定了 T 细胞对发热和炎症的反应
热是炎症的一个主要特征,但它对免疫细胞的影响仍不确定。我们的研究表明,中度发热温度(39°C)会增加小鼠 CD4 T 细胞的新陈代谢、增殖和炎症效应活性,同时降低调节性 T 细胞的抑制能力。然而,受热的 T 辅助细胞 1(T H 1)会选择性地出现线粒体应激和 DNA 损伤,从而激活 Trp53 和干扰素基因刺激因子通路。虽然在这种温度下许多 T H 1 细胞死亡,但存活下来的 T H 1 细胞线粒体质量增加,活性增强。电子传递链复合物 1(ETC1)在发热范围的温度下迅速受损,这种现象对 T H 1 细胞特别不利。在人类慢性炎症中也检测到 T H 1 细胞的 DNA 损伤和 ETC1 标志升高。因此,发烧相关温度会破坏 ETC1,从而选择性地驱动 T H 1 细胞凋亡或适应,以保持基因组完整性并增强效应功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


