Two-dose priming immunization amplifies humoral immunity by synchronizing vaccine delivery with the germinal center response
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Prolonging exposure to subunit vaccines during the primary immune response enhances humoral immunity. Escalating-dose immunization (EDI), administering vaccines every other day in an increasing pattern over 2 weeks, is particularly effective but challenging to implement clinically. Here, using an HIV Env trimer/saponin adjuvant vaccine, we explored simplified EDI regimens and found that a two-shot regimen administering 20% of the vaccine followed by the remaining 80% of the dose 7 days later increased TFH responses 6-fold, antigen-specific germinal center (GC) B cells 10-fold, and serum antibody titers 10-fold compared with bolus immunization. Computational modeling of TFH priming and the GC response suggested that enhanced activation/antigen loading on dendritic cells and increased capture of antigen delivered in the second dose by follicular dendritic cells contribute to these effects, predictions we verified experimentally. These results suggest that a two-shot priming approach can be used to substantially enhance responses to subunit vaccines.
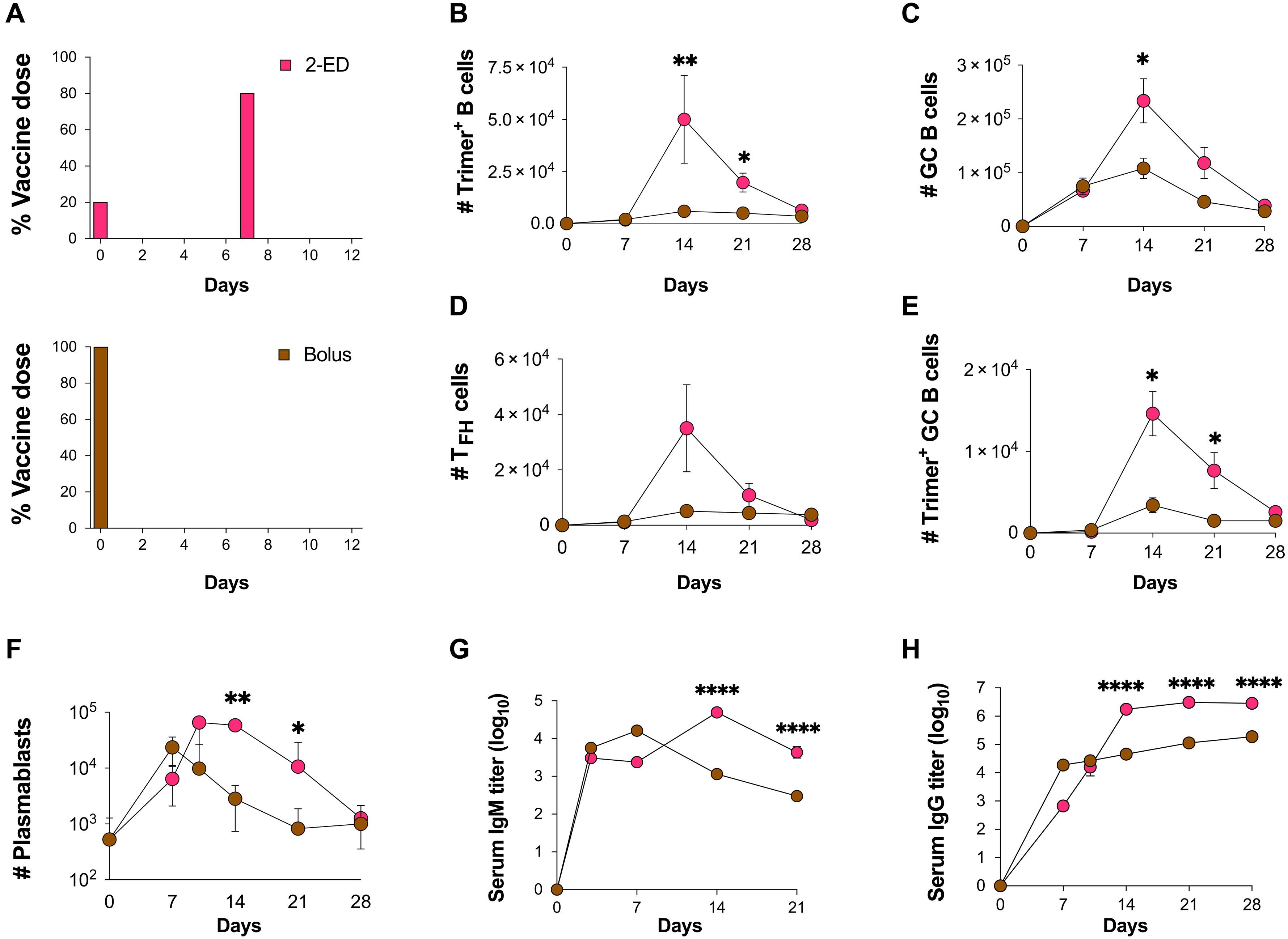
两剂启动免疫通过使疫苗接种与生殖中心反应同步,扩大体液免疫。
在初级免疫反应期间延长亚单位疫苗的接种时间可增强体液免疫。递增剂量免疫接种(EDI)是指在两周内以递增的方式隔天接种疫苗,这种方法特别有效,但在临床上实施却很困难。在这里,我们使用一种 HIV Env 三聚体/皂素佐剂疫苗探索了简化的 EDI 方案,并发现与栓剂免疫法相比,先注射 20% 疫苗,7 天后再注射剩余 80% 疫苗的两针方案可使 TFH 反应增加 6 倍,抗原特异性生殖中心 (GC) B 细胞增加 10 倍,血清抗体滴度增加 10 倍。TFH启动和GC反应的计算模型表明,树突状细胞的活化/抗原负载增强以及滤泡树突状细胞对第二剂抗原的捕获增加有助于产生这些效应,我们在实验中验证了这一预测。这些结果表明,两针引物法可以大大提高亚单位疫苗的应答。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


