Pd0 catalyst/carboxylic acid-mediated hydrofunctionalization of alkynes and allenes, two plausible hydropalladation mechanisms of a versatile process
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The review is focused on C–C and C–heteroatom bond forming methodologies involving reactions arising after hydropalladation of alkynes and allenes using Pd0 catalysts associated to catalytic or stoichiometric amounts of carboxylic acids. Most of the procedures occur with complete or high atom-economy, avoiding large generation of waste and producing functionalized compounds. The synthetic scope is presented and mechanistic problems are discussed.
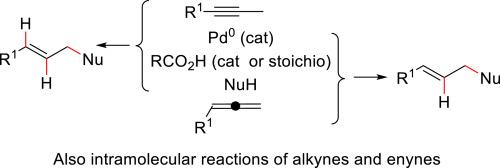
Pd0 催化剂/羧酸介导的炔烃和烯烃的加氢官能化--一种多功能工艺的两种似是而非的加氢钯化机制
本综述侧重于 C-C 和 C- 异原子键形成方法,涉及使用与催化或等量羧酸有关的钯催化剂对炔烃和烯烃进行加氢钯化后产生的反应。大多数过程都具有完全或高度的原子经济性,避免了大量废物的产生,并产生了功能化化合物。本文介绍了合成范围,并讨论了机制问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


