Intramolecular Silanoxy-Michael Reactions with Pendant Nitroalkenes: Racemic and Enantioselective
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
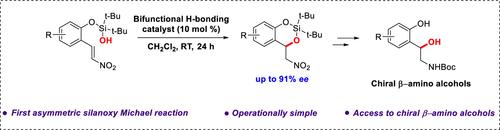
We present the first racemic and scalemic examples of di-tert-butyl silanoxy-Michael additions. Our operationally simple protocol is selective for nitro-olefins and simply involves stirring the substrate with an appropriate hydrogen-bond donor catalyst without any special precautions to exclude air or moisture. For each substrate examined, we have developed complementary protocols that optimize yield and enantioselectivity. Our reactions scale well, and the products are valuable intermediates for further transformations, including for the preparation of enantioenriched vicinal amino alcohols.
分子内硅烷氧-迈克尔反应与附硝基烯:外消旋和对映体选择性
我们首次提出了二叔丁基硅烷氧-迈克尔加成法的外消旋和缩放实例。我们的方案操作简单,对硝基烯烃具有选择性,只需将底物与适当的氢键供体催化剂一起搅拌即可,无需采取任何特殊的预防措施来排除空气或湿气。针对所研究的每种底物,我们都开发了可优化产率和对映体选择性的补充方案。我们的反应具有良好的规模,产物是进一步转化(包括制备对映体富集的代氨基醇)的重要中间体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


