Structural analysis of the TPI-Manchester, a thermolabile variant of human triosephosphate isomerase
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Human triosephosphate isomerase G122R, also known as TPI-Manchester, is a thermolabile variant detected in a screening of more than 3400 individuals from a population in Ann Arbor, Michigan. Here, the crystallographic structure of G122R was solved to determine the molecular basis of its thermal stability. Structural analysis revealed an increase in the flexibility of residues at the dimer interface, even though R122 is about 20 Å away, suggesting that long-range electrostatic interactions may play a key role in the mutation effect.
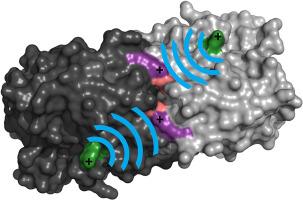
人类磷酸三糖异构酶热稳定性变体 TPI-Manchester 的结构分析
人类三磷酸异构酶 G122R(又称 TPI-Manchester)是在对密歇根州安阿伯市 3400 多人的筛选中发现的一种热稳定性变体。在此,我们解析了 G122R 的晶体学结构,以确定其热稳定性的分子基础。结构分析表明,尽管 R122 距二聚体界面约 20 Å,但二聚体界面处残基的柔韧性增加了,这表明长程静电相互作用可能在突变效应中起了关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


