HIF-1α knockdown attenuates inflammation and oxidative stress in ischemic stroke male rats via CXCR4/NF-κB pathway
Abstract
Background
Hypoxia inducible factor-1α (HIF-1α) is a sensitive indicator of oxygen homeostasis, of which the expression elevates following hypoxia/ischemia. This study reveals the specific mechanisms underlying the effects of HIF-1α on ischemic stroke (IS).
Methods
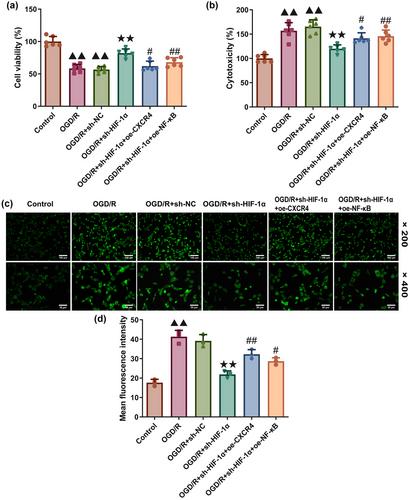
IS model was established using middle cerebral artery occlusion (MCAO)-modeled male rats and oxygen glucose deprivation/reoxygenation (OGD/R)-treated mice hippocampal cells HT22, followed by the silencing of HIF-1α and the overexpression of C-X-C motif chemokine receptor 4 (CXCR4) and nuclear factor-kappa B (NF-κB). Following the surgery, Garcia's grading scale was applied for neurological evaluation. Cerebral infarcts and injuries were visualized using 2,3,5-triphenyltetrazolium chloride and hematoxylin-eosin staining. The levels of tumor necrosis factor-α, Interleukin (IL)-6, IL-1β, malondialdehyde, and 8-hydroxy-2′-deoxyguanosine, were calculated via ELISA. MTT assay and lactate dehydrogenase (LDH) assay kit were adopted to determine the viability and cytotoxicity of OGD/R-modeled cells. Reactive oxygen species (ROS) generation was evaluated using a 2′-7′dichlorofluorescin diacetate (DCFH-DA) probe. The levels of HIF-1α, CXCR4, and NF-κB p65 were quantified via Western blot and immunofluorescence, respectively.
Results
HIF-1α knockdown improved Garcia's score, attenuated the cerebral infarct, inflammation, and ROS generation, and alleviated the levels of inflammatory cytokines and CXCR4/NF-κB p65 in MCAO-modeled rats. Such effects were reversed following the overexpression of CXCR4 and NF-κB. Also, in OGD/R-treated HT22 cells, HIF-1α silencing diminished the cytotoxicity and ROS production and reduced the expressions of CXCR4/NF-κB p65, while promoting viability. However, CXCR4/NF-κB p65 overexpression did the opposite.
Conclusion
HIF-1α knockdown alleviates inflammation and oxidative stress in IS through the CXCR4/NF-κB pathway.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


