Longitudinal DNA methylation in parent–infant pairs impacted by intergenerational social adversity: An RCT of the Michigan Model of Infant Mental Health Home Visiting
Abstract
Introduction
Early childhood development is a strong predictor of long-term health outcomes, potentially mediated via epigenetics (DNA methylation). The aim of the current study was to examine how childhood experiences, punitive parenting, and an intergenerational psychotherapeutic intervention may impact DNA methylation in young children and their mothers.
Methods
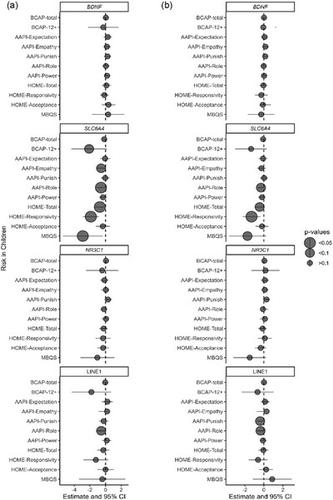
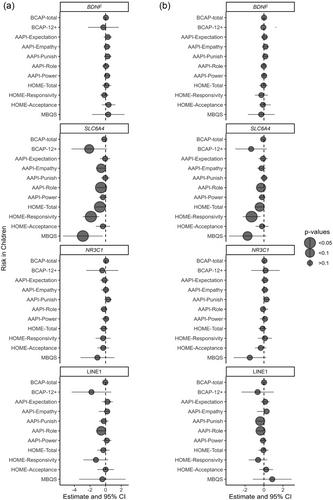
Mothers and their infants/toddlers between 0 and 24 months were recruited at baseline (n = 146, 73 pairs) to participate in a randomized control trial evaluating the effectiveness of The Michigan Model of Infant Mental Health Home Visiting (IMH-HV) parent–infant psychotherapy compared to treatment as usual. Baseline and 12-month post-enrollment data were collected in the family's home and included self-report questionnaires, biological saliva samples, home environment observation, video-taped parent–child interaction, and audio-recorded interviews. Saliva DNA methylation was measured at the genes, nuclear receptor subfamily 3 group C member 1 (NR3C1), solute carrier family 6 member 4 (SLC6A4), brain-derived neurotrophic factor (BDNF), and the genetic element, long interspersed nuclear element-1 (LINE1).
Results
For mothers, baseline methylation of BDNF, SLC6A4, NR3C1, or LINE1 was largely not associated with baseline measures of their childhood adversity, adverse life experiences, demographic characteristics related to structurally driven inequities, or to IMH-HV treatment effect. In infants, there were suggestions that methylation in SLC6A4 and LINE1 was associated with parenting attitudes. Infant BDNF methylation suggested an overall decrease in response to IMH-HV psychotherapy over 12 months.
Conclusions
Overall, our findings suggest that the epigenome in infants and young children may be sensitive to both early life experiences and parent–infant psychotherapy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


