Surface Nanostructuring of Copper Using Fluoride and Chloride
IF 3.5
4区 化学
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
Copper is an active electrocatalyst for various energy conversion reactions, but its performance depends on the structure of the active surface sites. In this work, we propose a simple strategy to tailor both the roughness and the active site's geometry of copper. To modify the surface of copper, we oxidize and reduce a copper polycrystalline electrode in 0.1 M solutions containing both sodium fluoride and sodium chloride with different chloride/fluoride molar ratios: (0.1‐x) M NaF+x M NaCl. To address the anion effect on the changes in surface geometry, we recorded the voltammetric fingerprints of the modified electrodes using lead underpotential deposition (UPD). The voltammetric analysis suggested that while chloride induces (n10) sites, fluoride promotes an increase in the active surface area and the growth of low‐coordinated sites with (110) or (111) geometry. Solutions containing both fluoride and chloride anions induced (n10) motifs covered by nanometric clusters, as observed by scanning electron microscopy, forming a highly defect‐rich surface. Our work provides a direct link between electrochemical response and ex‐situ structural characterization, and compares, in detail, the effect of chloride and fluoride on the surface nanostructuring of copper.
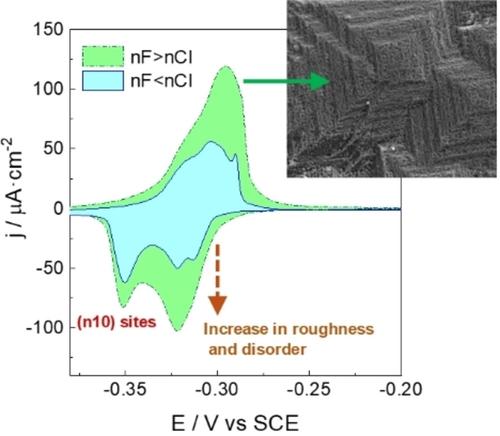
利用氟化物和氯化物对铜进行表面纳米结构处理
铜是一种活跃的电催化剂,可用于各种能量转换反应,但其性能取决于活性表面位点的结构。在这项工作中,我们提出了一种简单的策略来定制铜的粗糙度和活性位点的几何形状。为了改变铜的表面,我们在含有氟化钠和氯化钠的 0.1 M 溶液中对多晶铜电极进行氧化和还原,溶液中的氯/氟摩尔比各不相同:(0.1-x) M NaF+x M NaCl。为了研究阴离子对表面几何形状变化的影响,我们使用铅下电位沉积(UPD)技术记录了改性电极的伏安指纹。伏安分析表明,氯化物诱导了(n10)位点,而氟化物则促进了活性表面积的增加和具有(110)或(111)几何形状的低配位点的生长。扫描电子显微镜观察到,含有氟化物和氯化物阴离子的溶液诱导了纳米团簇覆盖的(n10)图案,形成了高度富缺陷的表面。我们的研究在电化学反应和原位结构表征之间建立了直接联系,并详细比较了氯化物和氟化物对铜表面纳米结构的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemElectroChem
ELECTROCHEMISTRY-
CiteScore
7.90
自引率
2.50%
发文量
515
审稿时长
1.2 months
期刊介绍:
ChemElectroChem is aimed to become a top-ranking electrochemistry journal for primary research papers and critical secondary information from authors across the world. The journal covers the entire scope of pure and applied electrochemistry, the latter encompassing (among others) energy applications, electrochemistry at interfaces (including surfaces), photoelectrochemistry and bioelectrochemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


