Crystal Facet Regulation and Ru Incorporation of Co3O4 for Acidic Oxygen Evolution Reaction Electrocatalysis
IF 4.8
Q2 NANOSCIENCE & NANOTECHNOLOGY
引用次数: 0
Abstract
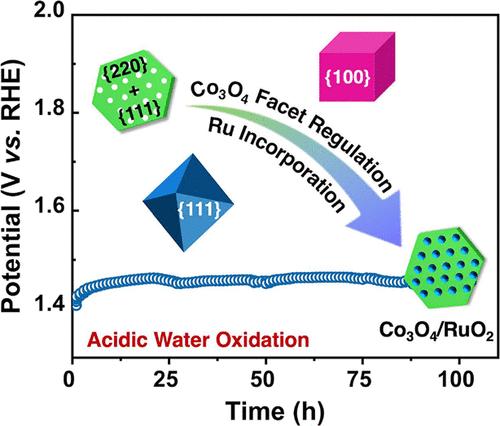
Acidic oxygen evolution reaction (OER) has long been the bottleneck of proton exchange membrane water electrolysis. Ru- and Ir-based oxides are currently state-of-the-art electrocatalysts for acidic OER, but their high cost limits their widespread application. Co3O4 is a promising alternative, yet the performance requires further improvement. Crystal facet engineering can effectively regulate the kinetics of surface electrochemistry and thus enhance the OER performance. However, the facet-dependent OER activity and corrosion behavior of Co3O4 have not been thoroughly studied. In this study, we systematically investigated the OER performance and crystal facet dependency of Co3O4. The results demonstrate that Co3O4 with mixed {111} and {110} facets exhibits better OER activity and stability than Co3O4 with {111} or {100} facets. The surface Co3+ species are responsible for the high OER activity, but its transformation to CoO2 is also the root cause of the dissolution, leading to an activity–stability trade-off effect. The possible approach to addressing this issue would be to increase the Co3+ contents by nanostructure engineering. To further improve the performance, Ru is introduced to the best-performing Co3O4. The resulting Co3O4/RuO2 heterostructure exhibits an overpotential of 257 mV at 10 mA cm–2 and can stably catalyze the OER for 100 h.
用于酸性氧进化反应电催化的 Co3O4 晶体面调节和 Ru 掺杂
长期以来,酸性氧进化反应(OER)一直是质子交换膜水电解的瓶颈。基于 Ru 和 Ir 的氧化物是目前最先进的酸性 OER 电催化剂,但其高昂的成本限制了其广泛应用。Co3O4 是一种很有前途的替代品,但其性能还需要进一步提高。晶面工程可以有效调节表面电化学动力学,从而提高 OER 性能。然而,人们对 Co3O4 晶面依赖性 OER 活性和腐蚀行为的研究还不够深入。在本研究中,我们系统地研究了 Co3O4 的 OER 性能和晶面依赖性。结果表明,具有{111}和{110}混合晶面的 Co3O4 比具有{111}或{100}晶面的 Co3O4 表现出更好的 OER 活性和稳定性。表面 Co3+ 物种是产生高 OER 活性的原因,但其向 CoO2 的转化也是导致溶解的根本原因,从而产生了活性-稳定性权衡效应。解决这一问题的可行方法是通过纳米结构工程增加 Co3+ 的含量。为了进一步提高性能,在性能最好的 Co3O4 中引入了 Ru。由此产生的 Co3O4/RuO2 异质结构在 10 mA cm-2 时的过电位为 257 mV,可稳定催化 OER 100 小时。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nanoscience Au
材料科学、纳米科学-
CiteScore
4.20
自引率
0.00%
发文量
0
期刊介绍:
ACS Nanoscience Au is an open access journal that publishes original fundamental and applied research on nanoscience and nanotechnology research at the interfaces of chemistry biology medicine materials science physics and engineering.The journal publishes short letters comprehensive articles reviews and perspectives on all aspects of nanoscience and nanotechnology:synthesis assembly characterization theory modeling and simulation of nanostructures nanomaterials and nanoscale devicesdesign fabrication and applications of organic inorganic polymer hybrid and biological nanostructuresexperimental and theoretical studies of nanoscale chemical physical and biological phenomenamethods and tools for nanoscience and nanotechnologyself- and directed-assemblyzero- one- and two-dimensional materialsnanostructures and nano-engineered devices with advanced performancenanobiotechnologynanomedicine and nanotoxicologyACS Nanoscience Au also publishes original experimental and theoretical research of an applied nature that integrates knowledge in the areas of materials engineering physics bioscience and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


