Analysis of Brain Protein Stability Changes in a Mouse Model of Alzheimer’s Disease
IF 3.8
2区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
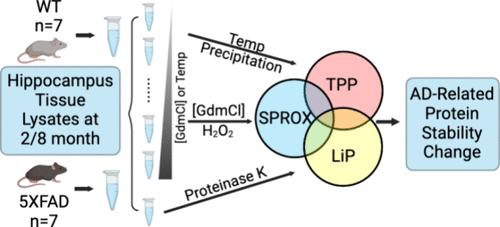
The stability of proteins from rates of oxidation (SPROX), thermal proteome profiling (TPP), and limited proteolysis (LiP) techniques were used to profile the stability of ∼2500 proteins in hippocampus tissue cell lysates from 2- and 8-months-old wild-type (C57BL/6J; n = 7) and transgenic (5XFAD; n = 7) mice with five Alzheimer’s disease (AD)-linked mutations. Approximately 200–500 protein hits with AD-related stability changes were detected by each technique at each age point. The hit overlap from technique to technique was low, and all of the techniques generated protein hits that were more numerous and largely different from those identified in protein expression level analyses, which were also performed here. The hit proteins identified by each technique were enriched in a number of the same pathways and biological processes, many with known connections to AD. The protein stability hits included 25 high-value conformation biomarkers with AD-related stability changes detected using at least 2 techniques at both age points. Also discovered were subunit- and age-specific AD-related stability changes in the proteasome, which had reduced function at both age points. The different folding stability profiles of the proteasome at the two age points are consistent with a different mechanism for proteasome dysfunction at the early and late stages of AD.
阿尔茨海默病小鼠模型脑蛋白稳定性变化分析
利用氧化率(SPROX)、热蛋白质组图谱(TPP)和有限蛋白质分解(LiP)技术分析了2个月和8个月大的野生型小鼠(C57BL/6J;n = 7)和具有5种阿尔茨海默病(AD)相关突变的转基因小鼠(5XFAD;n = 7)海马组织细胞裂解物中2500个蛋白质的稳定性。在每个年龄段,每种技术都能检测到约 200-500 个与阿尔茨海默病相关的稳定性变化蛋白。不同技术之间的命中重叠率很低,而且所有技术产生的蛋白命中数量更多,与蛋白表达水平分析中发现的蛋白有很大不同,而蛋白表达水平分析也在此进行。每种技术所发现的命中蛋白质都富集在一些相同的通路和生物过程中,其中许多与AD有已知的联系。蛋白质稳定性命中蛋白包括25个高价值构象生物标记物,在两个年龄点上至少使用两种技术检测到了与AD相关的稳定性变化。此外还发现了蛋白酶体亚基和年龄特异性的与AD相关的稳定性变化,蛋白酶体在两个年龄点的功能都有所降低。蛋白酶体在两个年龄段不同的折叠稳定性特征与蛋白酶体在AD早期和晚期功能障碍的不同机制是一致的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Proteome Research
生物-生化研究方法
CiteScore
9.00
自引率
4.50%
发文量
251
审稿时长
3 months
期刊介绍:
Journal of Proteome Research publishes content encompassing all aspects of global protein analysis and function, including the dynamic aspects of genomics, spatio-temporal proteomics, metabonomics and metabolomics, clinical and agricultural proteomics, as well as advances in methodology including bioinformatics. The theme and emphasis is on a multidisciplinary approach to the life sciences through the synergy between the different types of "omics".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


