The role of interactions between the cationic backbone and basic anions in green and ultra-selective catalytic synthesis of ethyl methyl carbonate in tunable ionized frameworks
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
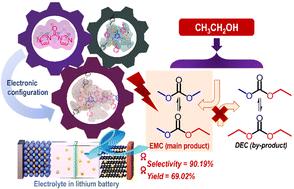
The significance of renewable energy sources underscores the importance of developing efficient battery technologies. Ethyl methyl carbonate (EMC), with its superior performance as an electrolyte, is widely utilized in lithium-ion batteries. However, the production of EMC through green transesterification of dimethyl carbonate (DMC) with ethanol encounters challenges due to low EMC selectivity and catalyst reusability issues. In this study, we present the initial instance of utilizing an imidazole-based ionic framework, [CPIL-M]n[PhO], which exhibits an interaction between its cationic backbone and basic anions. This interaction is specifically designed to catalyse the selective transformation of basic anions with suitable Lewis basicity in the production of EMC via transesterification. The introduction of N,N′-carbonyldiimidazole into the ionic framework allows for tunable modulation of Lewis basicity on an electronic level, enhancing catalytic activity without compromising selectivity. These innovative designs enable [CPIL-M]4[PhO] to exhibit remarkable performance, achieving 69.02% EMC yield and 90.19% selectivity, outperforming most reported catalysts. These findings could pave the way for the accelerated development of efficient catalysts for the sustainable production of EMC through transesterification methods in the future, thereby supporting the energy-efficient transition towards renewable energy sources.
阳离子骨架和碱性阴离子之间的相互作用在可调离子化框架中绿色和超选择性催化合成碳酸甲乙酯中的作用
可再生能源的重要性凸显了开发高效电池技术的重要性。碳酸甲乙酯(EMC)作为一种性能优越的电解质,被广泛应用于锂离子电池中。然而,通过碳酸二甲酯(DMC)与乙醇的绿色酯交换反应生产 EMC 时,由于 EMC 的选择性低和催化剂的可重复使用性问题而遇到了挑战。在本研究中,我们首次展示了利用咪唑类离子框架 [CPIL-M]n[PhO]的实例,该框架的阳离子骨架与碱性阴离子之间存在相互作用。这种相互作用专门用于催化具有适当路易斯碱性的碱性阴离子的选择性转化,通过酯交换反应生产 EMC。在离子框架中引入 N,N′-羰基二咪唑可以在电子层面上对路易斯碱性进行可调调节,从而在不影响选择性的情况下提高催化活性。这些创新设计使[CPIL-M]4[PhO]表现出卓越的性能,实现了 69.02% 的 EMC 收率和 90.19% 的选择性,优于大多数已报道的催化剂。这些发现可为将来通过酯交换反应方法加速开发可持续生产 EMC 的高效催化剂铺平道路,从而支持向可再生能源的高能效过渡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


