Synthesis of α-methylene-δ-valerolactone and its selective polymerization from a product mixture for concurrent separation and polymer production†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
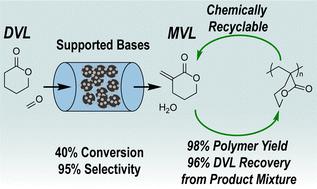
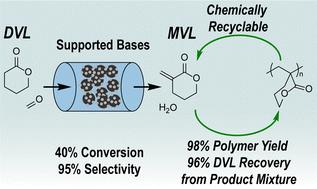
We report the continuous, gas-phase synthesis of α-methylene-δ-valerolactone (MVL) from δ-valerolactone (DVL) and formaldehyde (FA) over alkaline earth oxide catalysts. MgO, CaO, and BaO supported on silica (∼5 wt%) were active for MVL production (613 K, 0.4 kPa DVL, 1.2 kPa FA, 101 kPa total pressure). CaO and BaO showed 90% and 83% selectivity to MVL at ∼60% DVL conversion, respectively. Decreasing contact times improved MVL selectivity for all three catalysts, achieving near quantitative selectivity at DVL conversions <40% with CaO. Further studies with CaO indicated that increasing the FA partial pressure for a given DVL partial pressure negligibly changed conversion while maintaining high selectivity; however, increasing the reaction temperature generally resulted in lower MVL selectivity. Deactivation and carbon loss were attributed to non-volatile compound formation from series and parallel reactions that consume MVL and DVL and poison the catalyst surface. These side reactions were more pronounced at high temperatures and higher contact times. While slow deactivation poses a challenge, the catalyst could be fully regenerated by calcining at 773 K for 4 h under flowing air. As the product mixture of MVL and DVL is difficult to separate, we developed a selective polymerization strategy to convert either one or both monomers into valuable polymeric materials, thereby achieving efficient separation and concurrent polymer production. Using a model mixture of 30 wt% of MVL in DVL, vinyl-addition polymerization converted MVL to the corresponding vinyl polymer (PMVL)VAP in 98% yield, while DVL was recovered in 96% yield by distillation. Alternatively, ring-opening polymerization of the same mixture resulted in a DVL/MVL copolyester and separatable vinyl homopolymer P(MVL)VAP.
α-亚甲基-δ-戊内酯的合成及其从产品混合物中选择性聚合以同时进行分离和聚合物生产
我们报告了在碱土氧化物催化剂上由δ-戊内酯(DVL)和甲醛(FA)连续气相合成α-亚甲基-δ-戊内酯(MVL)的过程。以二氧化硅(5 wt%)为载体的氧化镁、氧化钙和氧化钡对生产 MVL 具有活性(613 K、0.4 kPa DVL、1.2 kPa FA、101 kPa 总压)。在 DVL 转化率为 60% 时,CaO 和 BaO 对 MVL 的选择性分别为 90% 和 83%。缩短接触时间可提高所有三种催化剂对 MVL 的选择性,CaO 在 DVL 转化率为 40% 时可达到接近定量的选择性。对 CaO 的进一步研究表明,在 DVL 分压一定的情况下,提高 FA 分压对转化率的影响可以忽略不计,同时还能保持较高的选择性;然而,提高反应温度通常会导致 MVL 选择性降低。失活和碳损失的原因是串联和并联反应形成的非挥发性化合物消耗了 MVL 和 DVL 并毒害了催化剂表面。这些副反应在高温和较长的接触时间下更为明显。虽然缓慢失活是一个挑战,但催化剂可以在流动空气中于 773 K 煅烧 4 小时后完全再生。由于 MVL 和 DVL 的产品混合物难以分离,我们开发了一种选择性聚合策略,将其中一种或两种单体转化为有价值的聚合物材料,从而实现高效分离并同时生产聚合物。使用 30 wt% MVL 与 DVL 的模型混合物,乙烯基加成聚合将 MVL 转化为相应的乙烯基聚合物 (PMVL)VAP,收率为 98%,而通过蒸馏回收的 DVL 收率为 96%。另外,对相同的混合物进行开环聚合,可得到 DVL/MVL 共聚酯和可分离的乙烯基均聚物 P(MVL)VAP。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


