Nationwide survey of the secondary findings in cancer genomic profiling: survey including liquid biopsy
IF 2.6
3区 生物学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
We surveyed the status of the secondary finding (SF) disclosure in comprehensive genome profiling (CGP) in 2020. The situation has changed: increase in the number of hospitals that provide CGP, an update to the Comprehensive Tumor Genomic Profiling: Materials for Review of Secondary Findings (CTGPMRSF), and the addition of a liquid biopsy test, FoundationOne® Liquid CDx (F1L). Moreover, the actual situation was unclear because the 2020 survey did not include all designated and cooperative hospitals. Herein, we conducted a questionnaire survey of all designated-core, designated, and cooperative hospitals to identify the current status and challenges concerning SF in the CGP in 2022. A total of 82.1% of the hospitals responded and 77.7% of the response was from cooperative hospitals. Approximately 80% of the hospitals used CTGPMRSF. SF disclosure, confirmatory test implementation, and SF confirmation rates were 12.4%, 31.6%, and 46.6% for FoundationOne® CDx (F1CDx), respectively, and 6.8%, 31.8%, and 70.7% for F1L, respectively. The implementation rate of the confirmatory test was substantially higher in hospitals with genetic experts and in hospitals that could conduct confirmatory tests on the same day. Our survey provides insight into how SF is handled in Japan. The percentage of cases leading to confirmatory tests has gradually increased, although challenges such as insurance coverage limitations and varied understanding of SF among patients and healthcare providers persist. With the increasing use of whole-genome analysis, our findings will provide valuable insights into establishing an effective SF disclosure system.
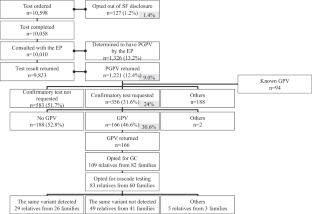

全国癌症基因组剖析二次发现调查:包括液体活检在内的调查
我们调查了2020年全面基因组剖析(CGP)中二次发现(SF)的披露情况。情况已经发生了变化:提供 CGP 的医院数量增加,《肿瘤基因组综合分析》(Comprehensive Tumor Genomic Profiling:次要结果审查材料》(CTGPMRSF)的更新,以及液体活检试验 FoundationOne® Liquid CDx (F1L) 的增加。此外,由于 2020 年的调查并未包括所有指定医院和合作医院,因此实际情况并不清楚。在此,我们对所有指定核心医院、指定医院和合作医院进行了问卷调查,以确定2022年CGP中有关SF的现状和挑战。共有 82.1% 的医院做出了回应,其中 77.7% 的回应来自合作医院。约 80% 的医院使用 CTGPMRSF。FoundationOne® CDx (F1CDx) 的 SF 披露率、确证试验实施率和 SF 确认率分别为 12.4%、31.6% 和 46.6%,F1L 的 SF 披露率、确证试验实施率和 SF 确认率分别为 6.8%、31.8% 和 70.7%。在拥有遗传专家的医院和能在同一天进行确证检验的医院,确证检验的实施率要高得多。我们的调查有助于了解日本如何处理 SF。尽管仍存在保险范围限制、患者和医疗服务提供者对 SF 的理解不同等挑战,但进行确证检验的病例比例已逐渐增加。随着全基因组分析的应用日益广泛,我们的调查结果将为建立有效的 SF 披露系统提供有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Human Genetics
生物-遗传学
CiteScore
7.20
自引率
0.00%
发文量
101
审稿时长
4-8 weeks
期刊介绍:
The Journal of Human Genetics is an international journal publishing articles on human genetics, including medical genetics and human genome analysis. It covers all aspects of human genetics, including molecular genetics, clinical genetics, behavioral genetics, immunogenetics, pharmacogenomics, population genetics, functional genomics, epigenetics, genetic counseling and gene therapy.
Articles on the following areas are especially welcome: genetic factors of monogenic and complex disorders, genome-wide association studies, genetic epidemiology, cancer genetics, personal genomics, genotype-phenotype relationships and genome diversity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


