Impacts of different hydrotropes on the aggregation behavior and physicochemical parameters of sodium dodecyl sulfate and ofloxacin drug mixture at several temperatures
Abstract
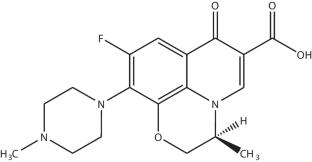
The study of the aggregation of anionic surfactant, sodium dodecyl sulfate (SDS) with an antibiotic drug, ofloxacin (OFC) has been performed employing conductivity measurement technique in different aq. hydrotropes (HDTs) media. The aqueous solutions of four HDTs (two anionic HDTs (sodium benzoate (NaBenz) and sodium salicylate (NaSal)) and two non-ionic HDTs (p-aminobenzoic acid (PABA) and resorcinol (ReSC))), having different compositions, were used to investigate their effect on the micellization phenomena of the SDS + OFC mixture. The micelle formation of the SDS + OFC mixture has been detected to be reliant on the nature and composition of additives as well as the temperature variation. At lower concentration (1.00 mmol kg−1) of HDTs in aq. media, the CMC values of the working system followed the following order: CMCPABA > CMCNaBenz > CMCReSC > CMCNaSal, and the CMC values increased with the rising concentration of HDTs. An increase in temperature at a fixed composition of OFC and HDTs resulted in the elevation of CMC values. The extent of counterion binding (β) values exhibited to be reduced with an increase of both concentration of HDTs and temperature. The negative free energy change (\({\Delta G}_{m}^{0}\)) for the micellization processes across all temperatures and investigated media indicates spontaneous micellization. The enthalpy change (\({\Delta H}_{m}^{0}\)) of micellization of the SDS + OFC mixture was obtained to be positive (endothermic) and negative (exothermic) at lower and higher temperatures in all media studied. The entropy change \({(\Delta S}_{m}^{0}\)) of micellization is positive even with varying temperatures in all the working additive media. The molar heat capacity (\({\Delta C}_{m,p}^{0}\)), transfer properties, and enthalpy-entropy compensation have been evaluated and explained with appropriate reasoning for the examined systems.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


