Optimizing Adoptive Cell Therapy for Solid Tumors via Epigenetic Regulation of T-cell Destiny
IF 10
2区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Adoptive cell therapy (ACT) emerged as a promising approach for cancer treatment, yet its application in solid tumors faced challenges such as inadequate tumor infiltration and cellular dysfunction. Histone acetylation is reported to play a crucial role in restoring T-cell function within tumor tissues. Building upon previous research, a novel strategy involving the co-loading of two drugs, G3C12 and vorinostat (SAHA), into PLGA microspheres to form G3C12+SAHA@PLGA is developed for intratumoral injection. The G3C12 peptide enhances adoptive T-cell recruitment to the tumor site by modulating the binding state of IFN-γ. While SAHA, a histone deacetylase inhibitor, promotes memory phenotypes of infiltrating T-cells and prevents their transition to an exhausted state. This synergistic approach effectively augmentes the efficacy of ACT in the “cold” tumor model (4T1) or the “hot” tumor model (CT26). These findings highlight the potential of combining epigenetic regulation with recruitment signaling as a means to enhance the therapeutic impact of ACT in treating solid tumors.
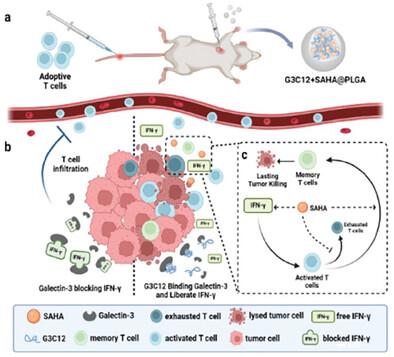
通过表观遗传调控 T 细胞命运优化实体瘤的适应性细胞疗法
适应性细胞疗法(ACT)是一种前景广阔的癌症治疗方法,但它在实体瘤中的应用面临着肿瘤浸润不足和细胞功能障碍等挑战。据报道,组蛋白乙酰化在恢复肿瘤组织内的 T 细胞功能方面起着至关重要的作用。在先前研究的基础上,我们开发了一种新策略,将两种药物(G3C12 和伏立诺他 (SAHA))共同载入 PLGA 微球,形成 G3C12+SAHA@PLGA 用于瘤内注射。G3C12 肽可通过调节 IFN-γ 的结合状态,增强肿瘤部位的采纳 T 细胞募集。而组蛋白去乙酰化酶抑制剂 SAHA 则能促进浸润 T 细胞的记忆表型,防止它们过渡到衰竭状态。这种协同方法有效增强了 ACT 在 "冷 "肿瘤模型(4T1)或 "热 "肿瘤模型(CT26)中的疗效。这些发现凸显了将表观遗传调控与招募信号结合起来作为一种手段来增强 ACT 在治疗实体瘤方面的疗效的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Healthcare Materials
工程技术-生物材料
CiteScore
14.40
自引率
3.00%
发文量
600
审稿时长
1.8 months
期刊介绍:
Advanced Healthcare Materials, a distinguished member of the esteemed Advanced portfolio, has been dedicated to disseminating cutting-edge research on materials, devices, and technologies for enhancing human well-being for over ten years. As a comprehensive journal, it encompasses a wide range of disciplines such as biomaterials, biointerfaces, nanomedicine and nanotechnology, tissue engineering, and regenerative medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


