Novel Ferrocene-Containing Pyrazole Analogs of Curcumin: Synthesis, Characterization, Antioxidant Activity, Cyclic Voltammetry, and In Vitro Biological Evaluation
IF 3.7
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
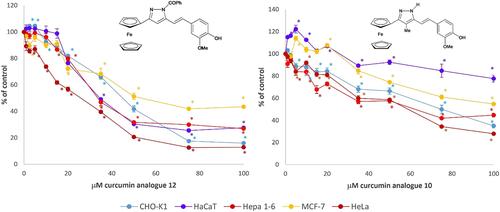
Structural modification is an advanced strategy to improve the biological activity of selected compounds and is effectively used to overcome the adverse properties of phytochemicals (e.g., curcumin), such as poor bioavailability and solubility, which limit their clinical use. Considering the fact that ferrocene is a very good candidate for organometallic derivatization of natural products, we have synthesized six new ferrocenylpyrazole analogs of curcumin 8–13. The new compounds were characterized by FT-IR, 1H, and 13C NMR spectroscopy and mass spectrometry (ESI-MS, HRMS), and their antioxidant and electrochemical properties as well as their biological activity were investigated. The best antioxidant activity evaluated with the DPPH assay was shown by compound 13, but it was lower than the activity achieved by curcumin. The prepared compounds showed no activity against the Gram-positive and Gram-negative bacteria tested, but the pyrazoles 8, 10, and 11 and the β-diketone precursors 5, 6, and 7 caused weaker inhibition of the growth of the yeast Candida utilis compared to nystatin. The higher antiproliferative activity in tumorous Hepa 1–6, HeLa, and MCF-7 cells compared to normal HaCaT and CHO-K1 cells was achieved by 10, 12, and 13. Compound 12 showed the strongest inhibitory effect in HeLa cells with an IC50 value of 25.20 μM, while the proliferation of normal cells was significantly less affected. In view of the higher selectivity index, compounds 10 and 12 could be considered for further research in the sense of therapeutic use in neoplasms of reproductive tissue.
姜黄素的新型含二茂铁吡唑类似物:合成、表征、抗氧化活性、环形伏安法和体外生物评估
结构修饰是提高特定化合物生物活性的先进策略,可有效克服植物化学物质(如姜黄素)生物利用度和溶解度低等限制其临床应用的不良特性。考虑到二茂铁是天然产物有机金属衍生化的良好候选物,我们合成了姜黄素 8-13 的六个新的二茂铁基吡唑类似物。我们利用傅立叶变换红外光谱、1H 和 13C NMR 光谱以及质谱(ESI-MS、HRMS)对这些新化合物进行了表征,并研究了它们的抗氧化性、电化学性质及其生物活性。用 DPPH 法评估,化合物 13 的抗氧化活性最佳,但低于姜黄素的活性。所制备的化合物对测试的革兰氏阳性菌和革兰氏阴性菌均无活性,但吡唑 8、10 和 11 以及 β-二酮前体 5、6 和 7 对念珠菌酵母生长的抑制作用弱于奈司他丁。与正常的 HaCaT 和 CHO-K1 细胞相比,10、12 和 13 对肿瘤 Hepa 1-6、HeLa 和 MCF-7 细胞的抗增殖活性更高。化合物 12 对 HeLa 细胞的抑制作用最强,IC50 值为 25.20 μM,而对正常细胞增殖的影响则明显较小。鉴于化合物 10 和 12 的选择性指数较高,可以考虑进一步研究它们对生殖组织肿瘤的治疗用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Organometallic Chemistry
化学-无机化学与核化学
CiteScore
7.80
自引率
10.30%
发文量
408
审稿时长
2.2 months
期刊介绍:
All new compounds should be satisfactorily identified and proof of their structure given according to generally accepted standards. Structural reports, such as papers exclusively dealing with synthesis and characterization, analytical techniques, or X-ray diffraction studies of metal-organic or organometallic compounds will not be considered. The editors reserve the right to refuse without peer review any manuscript that does not comply with the aims and scope of the journal. Applied Organometallic Chemistry publishes Full Papers, Reviews, Mini Reviews and Communications of scientific research in all areas of organometallic and metal-organic chemistry involving main group metals, transition metals, lanthanides and actinides. All contributions should contain an explicit application of novel compounds, for instance in materials science, nano science, catalysis, chemical vapour deposition, metal-mediated organic synthesis, polymers, bio-organometallics, metallo-therapy, metallo-diagnostics and medicine. Reviews of books covering aspects of the fields of focus are also published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


