IR and UV Spectroscopy of Gas-Phase Monohydrated Protonated Guanine
IF 2.7
2区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We use UV and infrared photodissociation spectroscopy to study monohydrated protonated guanine in a dual cryogenic ion trap spectrometer. The monohydrated complexes are formed through helium-mediated collisions between bare electrosprayed protonated guanine and low-pressure water vapor in a clustering trap maintained at 180 K, before being transferred to a quadrupole ion trap at 10 K. The spectrum of the monohydrated complex exhibits sharp vibronic transitions at the band origin and becomes broader and higher in intensity further in blue, which is very similar to protonated guanine but with a notable blue shift of ∼1850 cm–1 (∼0.23 eV). The UV hole-burning experiments showed that the vibronic bands recorded in the region of the band origin belong to a single conformer under our experimental conditions. The IR photodissociation spectrum in the 3000–3600 cm–1 range, with the aid of theoretical calculations (SCS-CC2/aug-cc-pVDZ), allowed us to assign the structure to the lowest energy N7–O conformer.
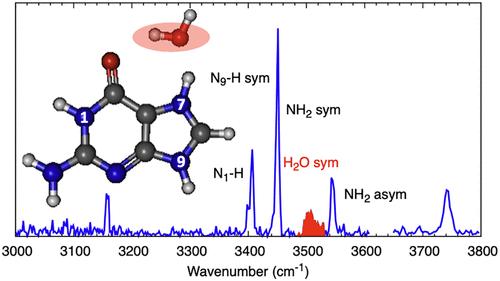
气相一水质子化鸟嘌呤的红外和紫外光谱分析
我们在双低温离子阱光谱仪中使用紫外和红外光解离光谱法研究单水合质子化鸟嘌呤。一水合复合物是通过氦介导的裸电喷质子化鸟嘌呤与低压水蒸气在一个保持在 180 K 的聚类阱中碰撞形成的,然后被转移到一个保持在 10 K 的四极离子阱中。一水合复合物的光谱在带源处呈现出尖锐的振子跃迁,蓝色更宽,强度更高,与质子化鸟嘌呤非常相似,但有明显的蓝色偏移 ∼1850 cm-1 (∼0.23 eV)。紫外空穴燃烧实验表明,在我们的实验条件下,在带源区域记录到的振子带属于单一构象。在理论计算(SCS-CC2/aug-cc-pVDZ)的帮助下,3000-3600 cm-1 范围内的红外光解离谱使我们能够将该结构归属于能量最低的 N7-O 构象。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry A
化学-物理:原子、分子和化学物理
CiteScore
5.20
自引率
10.30%
发文量
922
审稿时长
1.3 months
期刊介绍:
The Journal of Physical Chemistry A is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


