Effect of Electron-Donating Substituents and an Electric Field on the ΔEST of Selected Imidazopyridine Derivatives: A DFT Study
IF 2.8
2区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A series of thermally activated delayed fluorescent (TADF) molecules having an imidazopyridine acceptor, a benzene linker, and a 9,9-dimethyl-9,10-dihydroacridine donor are designed and examined using a quantum chemical approach. The above framework spatially separates the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), minimizing their overlap, ultimately resulting in a reduced energy gap between the excited singlet and triplet states (ΔEST). The impact of electron-donating substituents (-Me, -Et, -t-Bu, -OMe, and -NMe2) on the donor moiety of the parent molecule 2-(4-(9,9-dimethylacridin-10(9H)-yl)phenyl)imidazo[1,2-a]pyridine-3,6-dicarbonitrile (Ac-CNImPy) is investigated. The calculated results revealed that for a given substituent, the para-substituted derivatives exhibit relatively less ΔEST, compared to that of the respective ortho- and meta derivatives. The value of ΔEST decreased with an increase in the electron-donating capacity of the substituent. Additionally, the ΔEST of the disubstituted derivatives is found to be less than that for the monosubstituted derivatives. The charge transport studies revealed that molecules with strong electron-donating substituents act as electron transporters. The effect of an external electric field (EEF) on ΔEST of the parent molecule Ac-CNIMPY and its derivative is also examined and revealed that the ΔEST can be further reduced by applying an electric field of appropriate strength in a direction perpendicular to the dipole moment of the molecule and in the plane of the acceptor moiety.
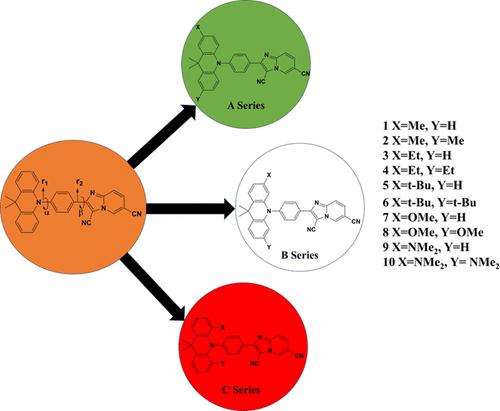
电子捐赠取代基和电场对选定咪唑吡啶衍生物 ΔEST 的影响:DFT 研究
本研究采用量子化学方法设计并研究了一系列热激活延迟荧光(TADF)分子,这些分子具有咪唑吡啶受体、苯连接体和 9,9 二甲基-9,10-二氢吖啶供体。上述框架在空间上分离了最高占位分子轨道(HOMO)和最低未占位分子轨道(LUMO),最大限度地减少了它们之间的重叠,最终导致激发的单重态和三重态之间的能隙(ΔEST)减小。研究了电子供体取代基(-Me、-Et、-t-Bu、-OMe 和 -NMe2)对母体分子 2-(4-(9,9-二甲基吖啶-10(9H)-基)苯基)咪唑并[1,2-a]吡啶-3,6-二腈(Ac-CNImPy)供体分子的影响。计算结果表明,对于给定的取代基,对位取代的衍生物表现出的 ΔEST 值相对低于正代和偏代衍生物。随着取代基给电子能力的增加,ΔEST 值也随之降低。此外,还发现二取代衍生物的 ΔEST 值小于单取代衍生物。电荷传输研究表明,具有强电子捐赠取代基的分子具有电子传输功能。研究还考察了外加电场(EEF)对母体分子 Ac-CNIMPY 及其衍生物的ΔEST 的影响,结果表明,在垂直于分子偶极矩的方向和受体分子的平面上施加适当强度的电场,可以进一步降低ΔEST。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry A
化学-物理:原子、分子和化学物理
CiteScore
5.20
自引率
10.30%
发文量
922
审稿时长
1.3 months
期刊介绍:
The Journal of Physical Chemistry A is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


