Unraveling Chlorite Oxidation Pathways in Equatorially Heteroatom-Substituted Nonheme Iron Complexes
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
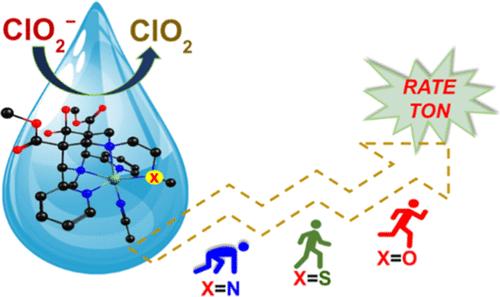
The first-coordination sphere of catalysts is known to play a crucial role in reaction mechanisms, but details of how equatorial ligands influence the reactivity remain unknown. Heteroatom ligated to the equatorial position of iron centers in nonheme iron metalloenzymes modulates structure and reactivity. To investigate the impact of equatorial heteroatom substitution on chlorite oxidation, we synthesized and characterized three novel mononuclear nonheme iron(II) complexes with a pentadentate bispidine scaffold. These complexes feature systematic substitutions at the equatorial position in the bispidine ligand framework where the pyridine group is replaced with NMe2, SMe, and OMe groups. The three iron(II)–bispidine complexes were subjected to studies in chlorite oxidation reactions as a model pathway for oxygen atom transfer. Chlorine oxyanions, which have the halide in an oxidation state ranging from +1 to +7, have numerous applications but can contaminate water bodies, and this demands urgent environmental remediation. Chlorite, a common precursor to chlorine dioxide, is of particular interest due to the superior antimicrobial activity of chlorine dioxide. Moreover, its generation leads to fewer harmful byproducts in water treatment. Here, we demonstrate that these complexes can produce chlorine dioxide from chlorite in acetate buffer at room temperature and pH 5.0, oxidizing chlorite through the in situ formation of high-valent iron(IV)–oxo intermediates. This study establishes how subtle changes in the coordination sphere around iron can influence the reactivity.
揭示赤道异构体取代的非血红素铁络合物中的亚氯酸盐氧化途径
众所周知,催化剂的第一配位层在反应机制中起着至关重要的作用,但赤道配体如何影响反应活性的细节仍不为人知。与非血红素铁金属酶中铁中心的赤道位置相连的杂原子会改变结构和反应活性。为了研究赤道杂原子置换对亚氯酸盐氧化的影响,我们合成并鉴定了三种具有五价双脒支架的新型单核非血红素铁(II)配合物。这些配合物的特点是在双脒配体框架的赤道位置进行了系统取代,其中吡啶基被 NMe2、SMe 和 OMe 基团取代。这三种铁(II)-双脒配合物在作为氧原子转移模型途径的亚氯酸盐氧化反应中进行了研究。氯氧阴离子的卤化物氧化态为 +1 至 +7,用途广泛,但会污染水体,因此急需进行环境修复。由于二氧化氯具有卓越的抗菌活性,二氧化氯的常见前体--亚氯酸盐尤其引人关注。此外,二氧化氯的生成还能减少水处理过程中的有害副产品。在这里,我们证明了这些复合物可以在室温和 pH 值为 5.0 的醋酸盐缓冲液中从亚氯酸盐中生成二氧化氯,通过原位形成高价铁(IV)-氧中间体来氧化亚氯酸盐。这项研究证实了铁周围配位层的微妙变化是如何影响反应活性的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


