Mechanochemical Synthesis of Manganese-Modified Microscale Zerovalent Iron for Efficient Cr(VI) Removal: Performance and Mechanism
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
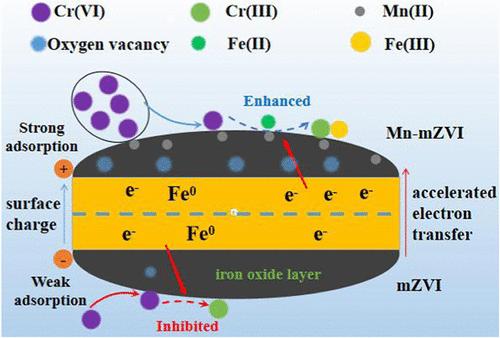
Metal doping for improving the reactivity of zerovalent iron (ZVI) has been well studied, while Mn(II)-modified microscale ZVI (Mn-mZVI) has not yet been explored. Herein, ball-milled Mn-mZVI was fabricated and used for Cr(VI) removal. Characterization analysis showed that the structure, composition, and charge of mZVI changed after the Mn(II) modification. The comparative test showed that Mn-mZVI could remove 100% of Cr(VI) within 10 min, whereas mZVI removed negligible Cr(VI) within 60 min. The zeta-potential and electrochemical evidence verified that the enhanced electrostatic attraction and electron-transfer ability contributed to the superior Cr(VI) removal performance of Mn-mZVI. Moreover, the solution pH increase caused the decline of Cr(VI) removal, and the presence of NO3– inhibited Cr(VI) removal, whereas other coexisting ions showed little influence on the Cr(VI) removal performance of Mn-mZVI. Chemical and material characterization analyses revealed that Cr(VI) reduction by Mn-mZVI was the combined action of Fe(0) and generated Fe(II). In addition, the reusability of Mn-mZVI was not ideal due to the surface passivation and loss of Mn(II), but the reactivity could be reactivated by ball-milling the reacted Mn-mZVI again with Mn(II). Overall, this work provides a new mentality for mZVI modification and is important to develop promising mZVI-based materials for Cr(VI) pollution control.
用于高效去除六价铬的锰改性微尺度零价铁的机械化学合成:性能和机理
掺杂金属以提高零价铁(ZVI)反应活性的研究已经很多,但锰(II)修饰的微尺度 ZVI(Mn-mZVI)尚未得到探索。本文制备了球磨 Mn-mZVI 并将其用于去除六价铬。表征分析表明,锰(II)改性后,mZVI 的结构、组成和电荷发生了变化。对比试验表明,Mn-mZVI 可在 10 分钟内去除 100%的六价铬,而 mZVI 可在 60 分钟内去除微量的六价铬。zeta电位和电化学证据证实,静电吸引和电子转移能力的增强是 Mn-mZVI 具有优异的六价铬去除性能的原因。此外,溶液 pH 值升高会导致六价铬去除率下降,NO3- 的存在会抑制六价铬的去除,而其他共存离子对 Mn-mZVI 的六价铬去除性能影响不大。化学和材料表征分析表明,Mn-mZVI 对 Cr(VI) 的还原是由 Fe(0) 和生成的 Fe(II) 共同作用的结果。此外,由于 Mn-mZVI 的表面钝化和 Mn(II) 的损失,Mn-mZVI 的可重复使用性并不理想,但可以通过球磨反应后的 Mn-mZVI 与 Mn(II) 重新激活反应活性。总之,这项工作为 mZVI 的改性提供了一种新思路,对于开发有前景的 mZVI 基材料用于六价铬污染控制具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
文献相关原料
公司名称
产品信息
阿拉丁
Commercial iron powder
阿拉丁
manganese chloride tetrahydrate
阿拉丁
potassium dichromate
阿拉丁
1,10-phenanthroline
阿拉丁
acetic acid
阿拉丁
ammonium acetate
阿拉丁
1,5-diphenylcarbazide
阿拉丁
hydrochloric acid
阿拉丁
sulfuric acid
阿拉丁
sodium chloride
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


