Oxygen Functionalization of Carbon Nanotubes Shifted the Formation Pathway of Hydroxyl Radicals in Catalytic Ozonation: The Overlooked Role of Hydrogen Peroxide
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
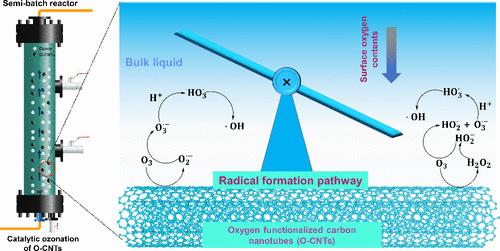
The oxygen functionalization of multiwalled carbon nanotubes (CNTs) could enhance their reactivity in catalytic ozonation for hydroxyl radical (•OH) formation. However, the detailed pathway for the transformation of ozone to •OH and the mechanism for the decreased treatment performance at acidic pH values remain unclear. In this study, surface oxygen-functionalized CNTs (O-CNTs) were prepared and used in catalytic ozonation to reveal the pathway for •OH formation. The efficiencies of ozone utilization and its conversion to •OH were increased by 2.7 and 554.8 times, respectively, under the catalysis of the O-CNTs. The great reactivity of the O-CNTs was related to their high surface oxygen contents and increased dispersion. Hydrogen peroxide was generated as a significant intermediate during the catalytic ozonation of the O-CNTs. The exposure of this substance linearly correlated with •OH exposure and pollutant degradation constants, with correlation coefficients of 0.991 and 0.911, respectively. The formation of hydrogen peroxide was relatively slower at acidic pH values, which explains the low performance of catalytic ozonation. A mechanism was proposed that involved the generation of hydrogen peroxide to trigger the peroxone process for free •OH formation. These findings deepen our understanding of oxygen functionalization and offer insights into the catalytic ozonation of surface oxygen-rich carbonaceous materials.
碳纳米管的氧官能化改变了催化臭氧中羟基自由基的形成途径:被忽视的过氧化氢的作用
多壁碳纳米管(CNTs)的氧官能化可以提高其在催化臭氧形成羟基自由基(-OH)时的反应能力。然而,臭氧转化为 -OH 的详细途径以及在酸性 pH 值下处理性能下降的机理仍不清楚。本研究制备了表面氧官能化的 CNTs(O-CNTs),并将其用于催化臭氧,以揭示 -OH 的形成途径。在 O-CNT 催化下,臭氧利用率和臭氧转化为 -OH 的效率分别提高了 2.7 倍和 554.8 倍。O-CNT 的高反应活性与其表面氧含量高和分散度增加有关。过氧化氢是 O-CNT 催化臭氧过程中产生的重要中间产物。这种物质的暴露量与 -OH 暴露量和污染物降解常数呈线性相关,相关系数分别为 0.991 和 0.911。在酸性 pH 值下,过氧化氢的形成速度相对较慢,这也是臭氧催化性能较低的原因。有人提出了一种机理,即过氧化氢的生成触发了过酮过程,从而形成游离 -OH。这些发现加深了我们对氧官能化的理解,并为表面富氧碳质材料的催化臭氧氧化提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


