Iron Enhancing Superoxide-Mediated Mn(II) Oxidation by Peroxymonosulfate: Elucidating the Role of Superoxide Radicals
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
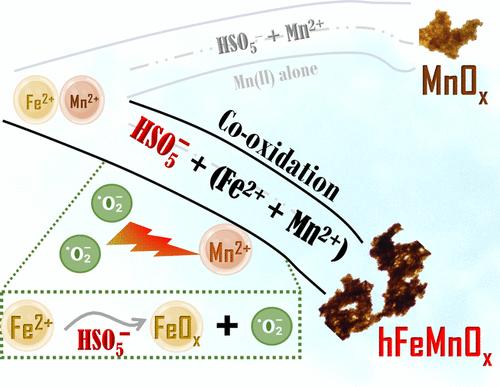
The effective removal of soluble Fe(II) and Mn(II) is problematic in water supply utilities. This study explored the oxidation behavior, kinetics, and reaction mechanisms of using peroxymonosulfate (PMS) to mediate the co-oxidation of Fe(II) and Mn(II) in natural water. At [Fe(II)] and [Mn(II)] of 1 mg/L, PMS oxidized all Fe(II) spontaneously within 15 s, irrespective of the oxidant concentration (50–500 μM) and solution pH (6–9), while it required 7–30 min for complete Mn(II) oxidation, indicating its distinctive behavior in reacting with Fe(II) and Mn(II). Scavenging assays and electron paramagnetic resonance (EPR) analysis revealed the dominant presence of O2•– in the system. EPR analysis combined with chemical probing experiments using nitroblue tetrazolium chloride suggested that O2•– was produced exclusively via surface reactions of ferric oxide with PMS. PMS co-oxidation eventually yielded amorphous hydrous manganese-bearing ferric co-oxides (hMnFeOx), with increasing Mn:Fe compositional ratios over time and pH, i.e., Mn0.31Fe0.69, Mn0.67Fe0.33, Mn0.93Fe0.07 at pH 7 and Mn0.68Fe0.32, Mn0.89Fe0.11, Mn0.90Fe0.10 at pH 9. The co-occurrence of Fe(II) provided hydrous FeOx surfaces enriched with chemisorbed oxygen (∼60%), acting as nucleation sites for the heterogeneous MnOx oxidation through enhanced electron transfer and surface complexation pathways. This co-occurrence thus reduced the half-life time of PMS-induced Mn(II) oxidation, by 5.3–18.7 times compared to the Mn(II) oxidation alone. This study provides fresh evidence, underscoring the significance of O2•– in PMS-mediated metal oxidation systems.
铁增强过氧化物介导的过一硫酸锰(II)氧化作用:阐明超氧自由基的作用
在供水设施中,如何有效去除可溶性铁(II)和锰(II)是一个难题。本研究探讨了使用过一硫酸盐(PMS)介导天然水中铁(II)和锰(II)共同氧化的氧化行为、动力学和反应机制。当[Fe(II)]和[Mn(II)]浓度为 1 mg/L 时,无论氧化剂浓度(50-500 μM)和溶液 pH 值(6-9)如何,PMS 都能在 15 秒内自发氧化所有的 Fe(II),而完全氧化 Mn(II) 则需要 7-30 分钟,这表明 PMS 与 Fe(II) 和 Mn(II) 的反应行为与众不同。清除测定和电子顺磁共振(EPR)分析表明,体系中主要存在 O2--。电子顺磁共振分析结合使用硝基蓝氯化四氮唑进行的化学探测实验表明,O2--完全是通过氧化铁与 PMS 的表面反应产生的。PMS 协同氧化最终产生了无定形的含锰铁协同氧化物(hMnFeOx),随着时间的推移和 pH 值的增加,锰:铁的成分比也在增加,即在 pH 值为 7 时,Mn0.31Fe0.69、Mn0.67Fe0.33、Mn0.93Fe0.07;在 pH 值为 9 时,Mn0.68Fe0.32、Mn0.89Fe0.11、Mn0.90Fe0.10。Fe(II)的共存提供了富含化学吸附氧(∼60%)的水合 FeOx 表面,通过增强的电子传递和表面络合途径成为异相 MnOx 氧化的成核位点。因此,与单独的 Mn(II)氧化相比,PMS 诱导的 Mn(II)氧化的半衰期缩短了 5.3-18.7 倍。这项研究提供了新的证据,强调了 O2 在 PMS 介导的金属氧化系统中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


