16TMCONF543: An Automatically Generated Data Set of Conformational Energies of Transition Metal Complexes Relevant to Catalysis
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
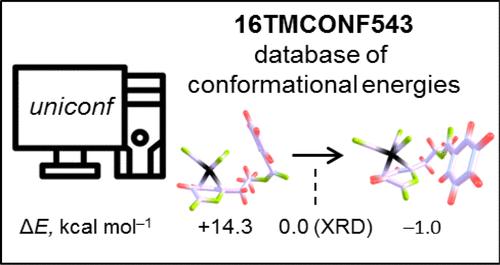
A database of conformational energies (CEs) of 16 transition metal (TM) complexes relevant to catalysis is automatically created by employing the new conformer generator program Uniconf. The generation procedure starts from an arbitrary high-energy structure and consistently produces conformer(s) similar to or even more energetically favorable than the optimized reference conformer retrieved from the Cambridge Structural Database. The reference CEs obtained with common dispersion-corrected functionals are employed to test the low-cost semiempirical methods. The superiority of the GFNn-xTB schemes over PM6*/7 methods is confirmed by the basic statistical analysis of the CEs. In addition, the influence of the vibrational thermostatistical (ΔGtherm) and continuum solvation (ΔGsolv) corrections on the CEs is examined in the framework of the (modified) scaled rigid-rotor-harmonic oscillator, (m)sRRHO, approximation, and solvation model based on density (SMD). In general, conformational Gibbs free energies exhibit excellent correlation with the respective electronic energies, especially if a more robust msRRHO scheme is employed for the calculation of ΔGtherm and in the case of a nonpolar solvent. The deviations from the perfect correlation occur if reduced CE windows of <5 kcal mol–1 are considered, implying a greater influence of these effects on the sorting of the low-energy structures.
16TMCONF543:自动生成的催化相关过渡金属配合物构象能数据集
通过使用新的构象生成程序 Uniconf,自动创建了与催化相关的 16 种过渡金属(TM)配合物的构象能(CE)数据库。该生成程序从任意高能结构开始,始终生成与从剑桥结构数据库中检索到的优化参考构象相似甚至更有利的构象。利用普通色散校正函数获得的参考构象,可以测试低成本的半经验方法。CE 的基本统计分析证实了 GFNn-xTB 方案优于 PM6*/7 方法。此外,在(修正的)比例刚性-转子-谐波振荡器(m)sRRHO 近似和基于密度的溶解模型(SMD)的框架内,研究了振动热统计(ΔGtherm)和连续溶解(ΔGsolv)修正对 CE 的影响。一般来说,构象吉布斯自由能与相应的电子能呈现出极佳的相关性,尤其是在采用更稳健的 msRRHO 方案计算 ΔGtherm 时,以及在非极性溶剂的情况下。如果考虑到 <5 kcal mol-1 的缩小 CE 窗口,则会出现与完美相关性的偏差,这意味着这些效应对低能结构排序的影响更大。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


