Synthesis, Characterization, and Catalytic Evaluation of Ruthenium Complexes Bearing Xanthinium-8-dithiocarboxylate Ligands Derived from Caffeine and Theophylline
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
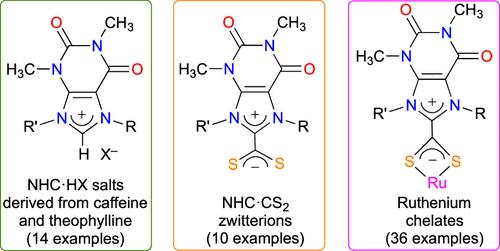
Various experimental procedures and purification techniques were applied to alkylate or arylate the N7 and N9 positions of caffeine and theophylline into xanthinium salts. These N-heterocyclic carbene (NHC) precursors were converted into xanthinium-8-dithiocarboxylate zwitterions using CS2 and either Cs2CO3 or NaOtBu. The NHC·CS2 betaines were employed as chelating ligands to prepare a wide variety of [RuX(p-cymene)(S2C·NHC)]Y (X = Cl, SAc; Y = Cl, PF6, [RuCl3(p-cymene)]) and [Ru(S2C·NHC)3]X2 (X = Cl, PF6) complexes that were characterized by NMR and HRMS. Moreover, the molecular structures of three betaines, one hetero-, and one homoleptic complex were determined by XRD. The catalytic potentials of all these complexes were investigated in the transfer hydrogenation of ketones with isopropanol, the synthesis of vinyl esters from benzoic acid and 1-hexyne, and the cyclopropanation of styrene with ethyl diazoacetate. The reduction of acetophenone into 1-phenylethanol was chosen as a model reaction for the former application. Monitoring the time course of this transformation showed that chelates bearing a NHC·CS2 ligand displayed an initial activity slightly higher than the analogous [RuCl2(p-cymene)(NHC)] complex. Contrastingly, for the last two catalytic processes, the Ru(S2C·NHC) chelates did not outperform their Ru–NHC counterparts.
含咖啡因和茶碱衍生的 8-二硫代羧酸黄嘌呤配体的钌配合物的合成、表征和催化评估
采用各种实验程序和纯化技术将咖啡因和茶碱的 N7 和 N9 位置烷基化或芳基化为黄嘌呤盐。使用 CS2 和 Cs2CO3 或 NaOtBu 将这些 N-杂环碳烯(NHC)前体转化为黄嘌呤鎓-8-二硫代羧酸盐齐聚物。NHC-CS2 betaines 被用作螯合配体,制备了多种[RuX(p-cymene)(S2C-NHC)]Y(X = Cl,SAc;Y = Cl,PF6,[RuCl3(p-cymene)])和[Ru(S2C-NHC)3]X2(X = Cl,PF6)配合物,并通过核磁共振和 HRMS 对其进行了表征。此外,还通过 XRD 确定了三种甜菜碱、一种杂环和一种同环配合物的分子结构。在酮与异丙醇的转移加氢反应、苯甲酸与 1-己炔合成乙烯基酯以及苯乙烯与重氮乙酸乙酯的环丙烷化反应中,研究了所有这些配合物的催化潜能。在前一种应用中,选择了将苯乙酮还原成 1-苯乙醇的模型反应。对这一转化过程的时间进程的监测表明,含有 NHC-CS2 配体的螯合物显示出的初始活性略高于类似的[RuCl2(p-cymene)(NHC)]配合物。相反,在后两个催化过程中,Ru(S2C-NHC)螯合物的活性并没有超过它们的 Ru-NHC 复合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


