Diabetes and Cognitive Decline: An Innovative Approach to Analyzing the Biophysical and Vibrational Properties of the Hippocampus
IF 3.7
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Diabetes Mellitus (DM) is a disease characterized by high blood glucose levels, known as hyperglycemia. Diabetes represents a risk factor for the development of neurodegenerative diseases, such as Alzheimer’s Disease (AD), one of the most prevalent neurodegenerative diseases worldwide, which leads to progressive mental, behavioral, and functional decline, affecting many brain structures, especially the hippocampus. Here, we aim to characterize the ultrastructural, nanomechanical, and vibrational changes in hyperglycemic hippocampal tissue using atomic force microscopy (AFM) and Raman spectroscopy. DM was induced in rats by streptozotocin injection (type 1) or dietary intervention (type 2). Cryosections of the hippocampus were prepared and analyzed on an MM8 AFM (Bruker) in Peak Force Quantitative Nanomechanics mode, performing 25 μm2 scans in 9 regions of 3 samples from each group. Ultrastructural and nanomechanical data such as surface roughness, area, volume, Young’s modulus, and adhesion were evaluated. The hippocampal samples were also analyzed on a T64000 Spectrometer (Horiba), using a laser λ = 632.8 nm, and for each sample, four spectra were obtained in different regions. AFM analyses show changes on the ultrastructural scale since diabetic animals had hippocampal tissue with greater roughness and volume. Meanwhile, diabetic tissues had decreased adhesion and Young’s modulus compared to control tissues. These were corroboratedby Raman data that shows changes in the molecular composition of diabetic tissues. The individual spectra show that the most significant changes are in the amide, cholesterol, and lipid bands. Overall, the data presented here show that hyperglycemia induces biophysical alterations in the hippocampal tissue of diabetic rats, providing novel biophysical and vibrational cues on the relationship between hyperglycemia and dementia.
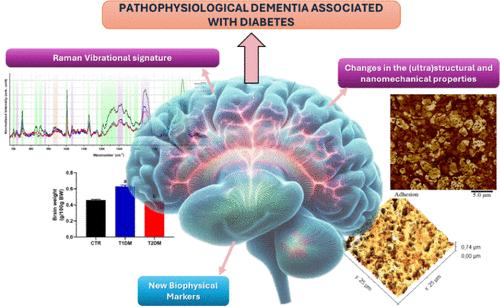
糖尿病与认知功能衰退分析海马体生物物理和振动特性的创新方法
糖尿病(DM)是一种以高血糖(即高血糖症)为特征的疾病。糖尿病是神经退行性疾病(如阿尔茨海默病(AD))的危险因素之一,而阿尔茨海默病是全球最普遍的神经退行性疾病之一,会导致智力、行为和功能的逐渐衰退,影响许多大脑结构,尤其是海马体。在这里,我们旨在利用原子力显微镜(AFM)和拉曼光谱表征高血糖海马组织的超微结构、纳米力学和振动变化。通过注射链脲佐菌素(类型 1)或饮食干预(类型 2)诱导大鼠患糖尿病。制备海马冷冻切片,并在MM8 AFM(布鲁克公司)的峰值力定量纳米力学模式下进行分析,对每组3个样本的9个区域进行25 μm2扫描。评估了超微结构和纳米力学数据,如表面粗糙度、面积、体积、杨氏模量和附着力。海马样本还在 T64000 光谱仪(Horiba)上进行了分析,使用的激光波长为 λ = 632.8 nm,每个样本在不同区域获得了四条光谱。原子力显微镜分析表明了超微结构的变化,因为糖尿病动物的海马组织更粗糙,体积更大。同时,与对照组织相比,糖尿病组织的粘附力和杨氏模量都有所下降。拉曼数据也证实了这一点,这些数据显示糖尿病组织的分子组成发生了变化。单个光谱显示,变化最显著的是酰胺、胆固醇和脂质带。总之,本文提供的数据表明,高血糖会诱导糖尿病大鼠海马组织发生生物物理改变,从而为研究高血糖与痴呆之间的关系提供了新的生物物理和振动线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Omega
Chemical Engineering-General Chemical Engineering
CiteScore
6.60
自引率
4.90%
发文量
3945
审稿时长
2.4 months
期刊介绍:
ACS Omega is an open-access global publication for scientific articles that describe new findings in chemistry and interfacing areas of science, without any perceived evaluation of immediate impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


