Glutaminase inhibition in combination with azacytidine in myelodysplastic syndromes: a phase 1b/2 clinical trial and correlative analyses
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
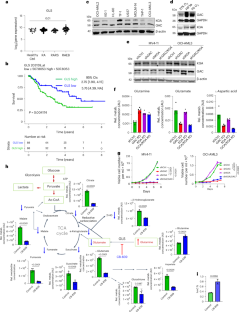
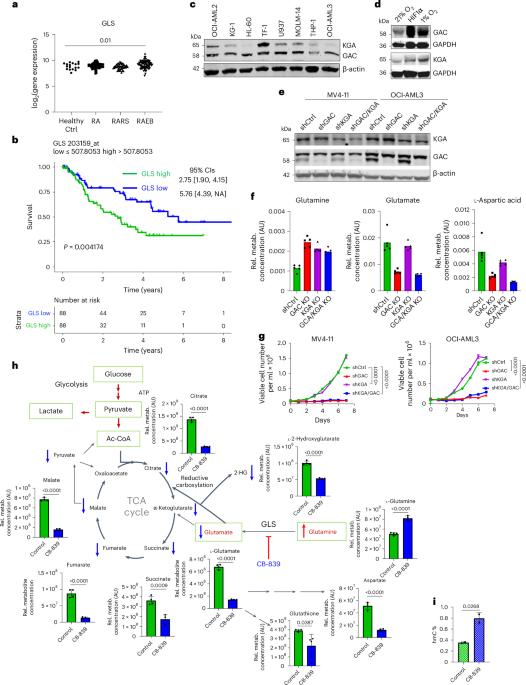
Malignancies are reliant on glutamine as an energy source and a facilitator of aberrant DNA methylation. We demonstrate preclinical synergy of telaglenastat (CB-839), a selective glutaminase inhibitor, combined with azacytidine (AZA), followed by a single-arm, open-label, phase 1b/2 study in persons with advanced myelodysplastic syndrome (MDS). The dual primary endpoints evaluated clinical activity, safety and tolerability; secondary endpoints evaluated pharmacokinetics, pharmacodynamics, overall survival, event-free survival and duration of response. The dose-escalation study included six participants and the dose-expansion study included 24 participants. Therapy was well tolerated and led to an objective response rate of 70% with (marrow) complete remission in 53% of participants and a median overall survival of 11.6 months, with evidence of myeloid differentiation in responders determined by single-cell RNA sequencing. Glutamine transporter solute carrier family 38 member 1 in MDS stem cells was associated with clinical responses and predictive of worse prognosis in a large MDS cohort. These data demonstrate the safety and efficacy of CB-839 and AZA as a combined metabolic and epigenetic approach in MDS. ClinicalTrials.gov identifier: NCT03047993 . DiNardo et al. perform a phase 1b/2 clinical trial of telaglenastat (CB-839) in combination with azacytidine in persons with advanced myelodysplastic syndromes and report on the treatment safety and efficacy, including a definition of clinical responders.
谷氨酰胺酶抑制剂联合氮杂胞苷治疗骨髓增生异常综合征:1b/2期临床试验及相关分析
恶性肿瘤依赖谷氨酰胺作为能量来源和异常 DNA 甲基化的促进剂。我们证明了选择性谷氨酰胺酶抑制剂 telaglenastat(CB-839)与氮杂胞苷(AZA)联用的临床前协同作用,随后在晚期骨髓增生异常综合征(MDS)患者中进行了单臂、开放标签、1b/2 期研究。双主要终点评估临床活性、安全性和耐受性;次要终点评估药代动力学、药效学、总生存期、无事件生存期和反应持续时间。剂量递增研究包括6名参与者,剂量扩大研究包括24名参与者。该疗法耐受性良好,客观反应率为70%,53%的参与者获得(骨髓)完全缓解,中位总生存期为11.6个月,单细胞RNA测序确定了反应者骨髓分化的证据。在一个大型MDS队列中,MDS干细胞中的谷氨酰胺转运体溶质运载家族38成员1与临床反应相关,并可预测较差的预后。这些数据证明了CB-839和AZA作为代谢与表观遗传联合疗法治疗MDS的安全性和有效性。ClinicalTrials.gov identifier:NCT03047993。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


