Tunneling Mechanisms of Quinones in Photosynthetic Reaction Center–Light Harvesting 1 Supercomplexes
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
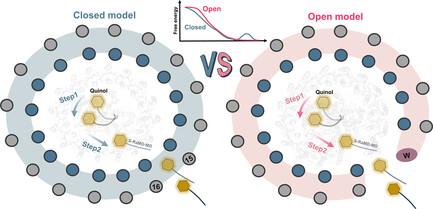
In photosynthesis, light energy is absorbed and transferred to the reaction center, ultimately leading to the reduction of quinone molecules through the electron transfer chain. The oxidation and reduction of quinones generate an electrochemical potential difference used for adenosine triphosphate synthesis. The trafficking of quinone/quinol molecules between electron transport components has been a long-standing question. Here, an atomic-level investigation into the molecular mechanism of quinol dissociation in the photosynthetic reaction center–light-harvesting complex 1 (RC–LH1) supercomplexes from Rhodopseudomonas palustris, using classical molecular dynamics (MD) simulations combined with self-random acceleration MD-MD simulations and umbrella sampling methods, is conducted. Results reveal a significant increase in the mobility of quinone molecules upon reduction within RC–LH1, which is accompanied by conformational modifications in the local protein environment. Quinol molecules have a tendency to escape from RC–LH1 in a tail-first mode, exhibiting channel selectivity, with distinct preferred dissociation pathways in the closed and open LH1 rings. Furthermore, comparative analysis of free energy profiles indicates that alternations in the protein environment accelerate the dissociation of quinol molecules through the open LH1 ring. In particular, aromatic amino acids form π-stacking interactions with the quinol headgroup, resembling the key components in a conveyor belt system. This study provides insights into the molecular mechanisms that govern quinone/quinol exchange in bacterial photosynthesis and lays the framework for tuning electron flow and energy conversion to improve metabolic performance.
光合作用反应中心-光收集 1 超级复合物中醌的隧道机制
在光合作用中,光能被吸收并传递到反应中心,最终通过电子传递链导致醌分子还原。醌的氧化和还原产生电化学电位差,用于合成三磷酸腺苷。醌/醌醇分子在电子传递元件之间的流动是一个长期存在的问题。本文采用经典分子动力学(MD)模拟结合自随机加速 MD-MD 模拟和伞状取样方法,从原子水平研究了来自浅色红假单胞菌(Rhodopseudomonas palustris)的光合反应中心-采光复合物 1(RC-LH1)超级复合物中醌解离的分子机制。结果表明,醌分子在 RC-LH1 内还原时的流动性显著增加,同时伴随着局部蛋白质环境的构象改变。醌分子倾向于以尾先模式从 RC-LH1 中逃逸,表现出通道选择性,在封闭和开放的 LH1 环中有不同的优先解离途径。此外,自由能曲线的比较分析表明,蛋白质环境的变化会加速醌醇分子通过开放的 LH1 环解离。特别是,芳香族氨基酸与醌头基团形成π堆叠相互作用,类似于传送带系统中的关键部件。这项研究深入揭示了支配细菌光合作用中醌/喹啉交换的分子机制,并为调整电子流和能量转换以提高代谢性能奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


