Inflammatory or Reparative? Tuning Macrophage Polarization Using Anodized Anisotropic Nanoporous Titanium Implant Surfaces
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
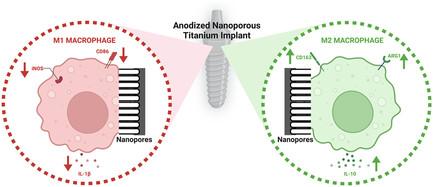
Modulating macrophage phenotype based on implant surface characteristics, including topography and chemistry, has been employed to enhance osseointegration and long-term functional outcomes for titanium (Ti)-based implants. An excessive and/or prolonged M1 macrophage response can lead to damaging immune-inflammatory reactions, negatively influencing the fate of the implant, and hence, modulating these responses via nanoscale implant surface modification is an emerging paradigm. Herein, an anodized titanium implant surface based on single-step electrochemical anodization, with preserved underlying microfeatures and superimposed nanopores (50 and 70 nm), compared with irregular rough and microrough (machined-like) surfaces, is investigated for its effect on the functions of primary macrophages in vitro. Significantly reduced macrophage proliferation and increased tissue-reparative M2 phenotype polarization are confirmed for the nanopores, which are more pronounced for 70 nm diameter. Moreover, osteoclastogenesis is reduced while osteogenic differentiation of osteoblasts is enhanced for the nanopores (higher for 70 nm pores). Advanced nanoengineered Ti implants can enhance titanium implant tissue integration by modulating the inflammatory response at the implant–cell interface.
炎症还是修复?利用阳极氧化各向异性纳米多孔钛种植体表面调节巨噬细胞极化
根据植入物表面特征(包括形貌和化学性质)调节巨噬细胞表型已被用于增强钛(Ti)基植入物的骨结合和长期功能效果。过度和/或长时间的 M1 巨噬细胞反应会导致破坏性免疫炎症反应,对植入物的命运产生负面影响,因此,通过纳米级植入物表面改性来调节这些反应是一种新兴的范例。本文研究了基于单步电化学阳极氧化处理的钛植入体表面,与不规则粗糙和微粗糙(类似机械加工)表面相比,该表面保留了底层微特征和叠加的纳米孔(50 纳米和 70 纳米),研究了其对体外原发性巨噬细胞功能的影响。结果表明,纳米孔明显减少了巨噬细胞的增殖,并增加了组织修复的 M2 表型极化,直径为 70 nm 的纳米孔效果更明显。此外,纳米孔减少了破骨细胞的生成,同时增强了成骨细胞的成骨分化(70 纳米孔的成骨分化更高)。先进的纳米工程钛植入物可以通过调节植入物-细胞界面的炎症反应来增强钛植入物的组织整合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


