A singly bonded gallanediyl with redox-active and redox-inert reactivity
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
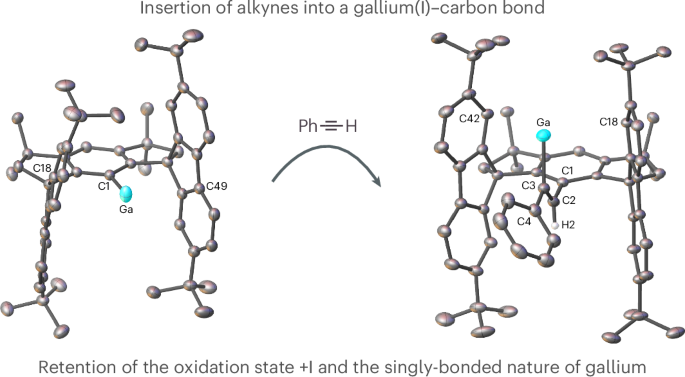
Singly bonded metallylenes (R–M:) of the group 13 elements feature a non-bonding pair of electrons together with two vacant orbitals, which makes them highly reactive ambiphiles that readily activate small molecules by oxidative addition. As a consequence of their pronounced reactivity, examples of organometallics with singly bonded aluminium(I) and gallium(I) centres remain rare. Here we report the one-step synthesis of a monomeric gallium(I) compound that readily undergoes oxidative addition reactions and, more remarkably, carbometalation reactions with alkynes by retention of the low +I oxidation state and the singly bonded nature of gallium. This observation contrasts with common reports on the reactivity of low-valent main-group compounds, which are regularly oxidized to compounds in a more stable higher oxidation state. This approach provides access to low-valent main-group compounds and paves the way for the development of bond-functionalization strategies that may enable the discovery of catalytic processes in the future. A singly bonded gallanediyl undergoes carbometalation reactions with alkynes by retaining the low +I oxidation state and the singly bonded nature of gallium. The insertion into the gallium(I)–carbon bond proceeds regioselectively and gives exclusively the syn-addition products.

具有氧化还原活性和氧化还原惰性的单键加兰二基
13 族元素中的单键偏铝烯(R-M:)具有一对非键电子和两个空闲轨道,这使它们成为高活性的ambiphiles,很容易通过氧化加成激活小分子。由于铝(I)和镓(I)具有明显的反应性,因此具有单键铝(I)和镓(I)中心的有机金属化合物仍然很少见。在这里,我们报告了一步合成单质镓(I)化合物的方法,这种化合物很容易发生氧化加成反应,更引人注目的是,通过保留低 +I 氧化态和镓的单键性质,它还能与炔烃发生碳甲基化反应。这一观察结果与有关低价主族化合物反应性的常见报道形成了鲜明对比,因为低价主族化合物经常被氧化成更稳定的高氧化态化合物。这种方法提供了获得低价主族化合物的途径,并为开发键功能化策略铺平了道路,这种策略可能会在未来促进催化过程的发现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


