Fabrication of Cu–CeO2 Catalyst with Abundant Interfacial Cu+–O–Ce3+–OV (Oxygen Vacancy) Sites for Boosting CO2 Electroreduction to Methane
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Accelerating the conversion of *CO to *CHO and promoting the adsorption and hydrogenation of *CHO are the keys to achieving a highly selective electrocatalytic CO2 reduction reaction (CO2RR) to CH4 over Cu-based catalysts. Herein, a novel electrocatalyst comprising highly dispersed Cu nanoclusters (CuNCs) supported on oxygen vacancy (OV)-rich CeO2 on carbon paper (CuNCs–CeO2/CP) with plentiful interfacial Cu+–O–Ce3+–OV sites is constructed via a facile electrodeposition method. Various in situ/ex situ characterizations and theoretical calculations unveil that the Cu+–O–Ce3+–OV sites can effectively regulate the pathway of the CO2RR, accelerate the *CO → *CHO process, stabilize the *CHO and *OCH3 intermediates, and promote their hydrogenation to produce CH4. Furthermore, the critical role of Ce3+ and the OV species in forming Cu+–O–Ce3+–OV to maintain the electrocatalytic CO2RR activity is revealed. The optimized CuNCs–CeO2/CP electrocatalyst exhibits a CH4 Faradaic efficiency of 68.3% at 500 mA cm–2, with a high partial current density of 340 mA cm–2.
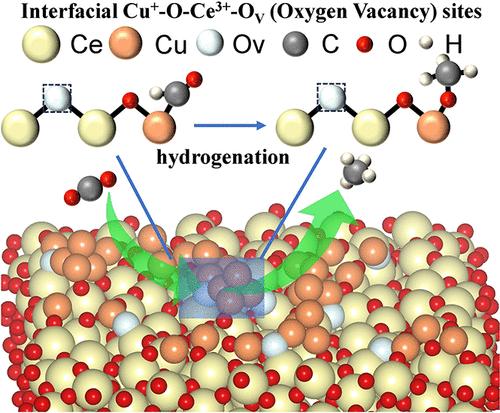
制备具有丰富表面 Cu+-O-Ce3+-OV(氧空位)位点的 Cu-CeO2 催化剂,促进 CO2 电还原为甲烷
加速*CO 向*CHO 的转化以及促进*CHO 的吸附和氢化是在铜基催化剂上实现高选择性电催化 CO2 还原反应(CO2RR)至 CH4 的关键。本文通过简便的电沉积方法,构建了一种新型电催化剂,该催化剂由高度分散的铜纳米团簇(CuNCs)支撑在碳纸上富含氧空位(OV)的CeO2(CuNCs-CeO2/CP)上,并具有大量的界面Cu+-O-Ce3+-OV位点。各种原位/原位表征和理论计算表明,Cu+-O-Ce3+-OV 位点能有效调节 CO2RR 的路径,加速 *CO → *CHO 过程,稳定 *CHO 和 *OCH3 中间产物,并促进它们加氢生成 CH4。此外,还揭示了 Ce3+ 和 OV 物种在形成 Cu+-O-Ce3+-OV 以保持 CO2RR 电催化活性方面的关键作用。优化后的 CuNCs-CeO2/CP 电催化剂在 500 mA cm-2 电流下的 CH4 法拉第效率为 68.3%,部分电流密度高达 340 mA cm-2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


