Tailoring Surface Electronic Structure of Spinel Co3O4 Oxide via Fe and Cu Substitution for Enhanced Oxygen Evolution Reaction
IF 9.6
1区 化学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Multimetal spinel oxides are promising candidates for the oxygen evolution reaction (OER) due to their ability to offer more accessible active sites and oxygen vacancies (Ovac). However, the utilization of redox-active species in spinel oxides is limited. Herein, we unveil an efficient multimetal spinel oxide using high-throughput methods. The oxide contains Fe and Cu substituted into Co sites following a stoichiometry of Fe0.6Cu0.6Co1.8O4. The dual cation substitution of Fe and Cu manipulates the electronic states and generates Ovac, thereby generating more accessible active species. This significantly improves the OH– adsorption capacity on spinel oxide triggering a more favorable OER reaction with a low overpotential of 265 mV at 10 mA cm–2 and high durability in an alkaline medium. Our work not only presents the utilization of a high-throughput approach to explore efficient catalysts with optimal composition but also provides useful insights into the modulation of electronic states for enhanced catalytic performance.
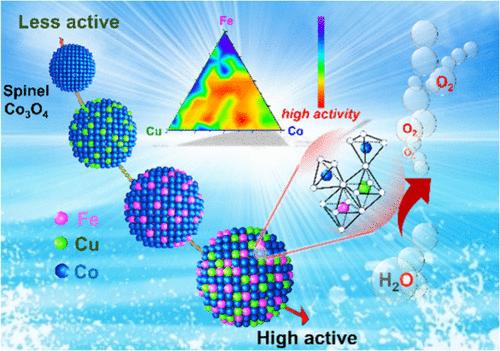
通过铁和铜置换调整尖晶石 Co3O4 氧化物的表面电子结构以增强氧进化反应
多金属尖晶石氧化物能够提供更多的活性位点和氧空位(Ovac),因此是氧气进化反应(OER)的理想候选物质。然而,尖晶石氧化物中氧化还原活性物种的利用率有限。在此,我们利用高通量方法揭示了一种高效的多金属尖晶石氧化物。该氧化物中的 Fe 和 Cu 按照 Fe0.6Cu0.6Co1.8O4 的化学计量取代了 Co 的位点。Fe和Cu的双阳离子置换操纵了电子状态并产生了Ovac,从而产生了更容易获得的活性物种。这大大提高了尖晶石氧化物对 OH- 的吸附能力,引发了更有利的 OER 反应,在 10 mA cm-2 时过电位低至 265 mV,并且在碱性介质中具有很高的耐久性。我们的工作不仅展示了利用高通量方法探索具有最佳组成的高效催化剂的方法,而且还为调控电子状态以提高催化性能提供了有益的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Materials Letters
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
14.60
自引率
3.50%
发文量
261
期刊介绍:
ACS Materials Letters is a journal that publishes high-quality and urgent papers at the forefront of fundamental and applied research in the field of materials science. It aims to bridge the gap between materials and other disciplines such as chemistry, engineering, and biology. The journal encourages multidisciplinary and innovative research that addresses global challenges. Papers submitted to ACS Materials Letters should clearly demonstrate the need for rapid disclosure of key results. The journal is interested in various areas including the design, synthesis, characterization, and evaluation of emerging materials, understanding the relationships between structure, property, and performance, as well as developing materials for applications in energy, environment, biomedical, electronics, and catalysis. The journal has a 2-year impact factor of 11.4 and is dedicated to publishing transformative materials research with fast processing times. The editors and staff of ACS Materials Letters actively participate in major scientific conferences and engage closely with readers and authors. The journal also maintains an active presence on social media to provide authors with greater visibility.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


