Donor-only substituted benzene achieves thermally activated delayed fluorescence
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Thermally activated delayed fluorescence (TADF) is a promising mechanism for harvesting triplet excitons in organic light-emitting diodes (OLEDs). The donor–acceptor (D–A) design is the most conventional strategy for developing efficient TADF emitters. A subsequently emerged approach, known as the multiple resonance (MR) effect, also employs electron-donating and electron-withdrawing functional groups. Thus, developing TADF materials has traditionally relied on ingenuity in selecting and combining two functional units. Here, we have realized a TADF molecule by utilizing only a carbazole donor moiety. This molecule is an unusual example in the family of TADF materials and offers better insight into the electronic structures in the excited states for luminescent materials. Thermally activated delayed fluorescence (TADF) is a promising mechanism for harvesting triplet excitons in organic light-emitting diodes, but TADF molecules typically rely on multiple functional units, such as both an electron donor and an electron acceptor. Here, the authors develop a TADF molecule using only benzene and carbazole donor moieties.
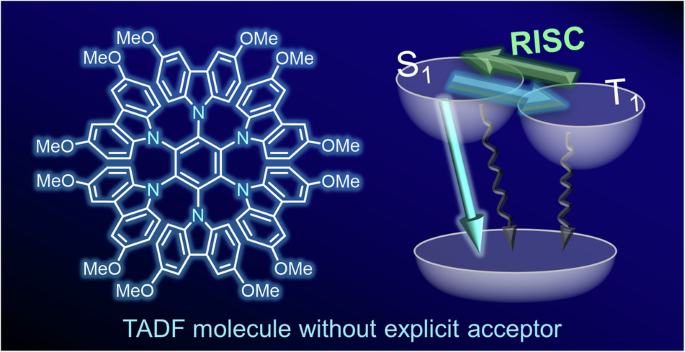
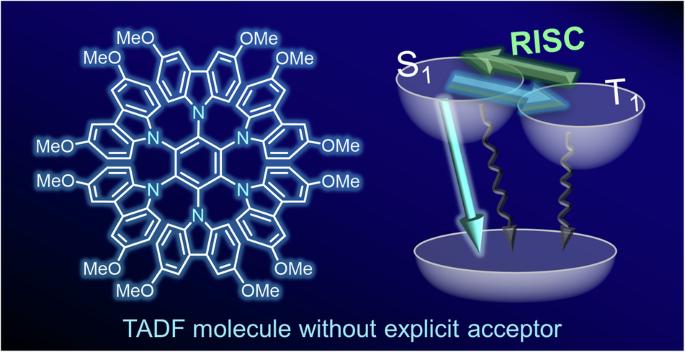
只有供体取代的苯实现热激活延迟荧光
热激活延迟荧光(TADF)是有机发光二极管(OLED)中一种很有前景的三重激子收集机制。供体-受体(D-A)设计是开发高效 TADF 发射器的最传统策略。随后出现的一种被称为多重共振(MR)效应的方法也采用了电子供体和电子吸附官能团。因此,TADF 材料的开发历来依赖于选择和组合两种功能单元的独创性。在这里,我们仅利用一个咔唑供体分子就实现了 TADF 分子。该分子是 TADF 材料家族中一个不同寻常的例子,它为我们深入了解发光材料激发态的电子结构提供了更多的线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


