Zinc tetrafluoroborate catalyzed α-stereoselective synthesis of pseudoglycals: efficient synthesis of digitoxin α-L-amicetose
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report here an efficient, fast, and cost-effective strategy for synthesizing pseudoglycals by the reaction of glycals with alcohols or nucleophiles using zinc tetrafluoroborate. This mild, transition metal-free approach allowed the α-selective synthesis of pseudoglycals using a wide range of acceptors containing various protecting groups/functionalities. This method is exemplified by the synthesis of digitoxin α-L-amicetose, a known potential cardiac glycoside anticancer agent. The improved 3-step synthesis from L-rhamnal afforded an overall yield of 54%, thus representing a significant improvement over the previous method.
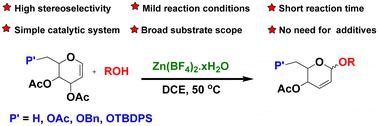
四氟硼酸锌催化α-严格选择性合成假甘氨酸:高效合成地高辛α-L-氨基乙糖
我们在此报告了一种高效、快速、经济的合成假甘氨酸的策略,该策略通过使用四氟硼酸锌使甘氨酸与醇或亲核物反应来合成假甘氨酸。这种温和、不含过渡金属的方法可以利用含有各种保护基团/官能团的多种受体,α 选择性地合成假甘氨酸。地高辛 α-L-amicetose 的合成就是这种方法的例证,它是一种已知的潜在强心苷抗癌剂。改进后的 L-鼠李糖三步合成法的总产率为 54%,比以前的方法有了显著提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


