Fluorescein-switching-based lateral flow assay for the detection of microRNAs†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Lateral flow assays (LFAs) are a cost-effective and rapid colorimetric technology that can be effectively used for nucleic acid tests (NATs) in various fields such as medical diagnostics and biotechnology. Given their importance, developing more diverse LFAs that operate through novel working mechanisms is essential for designing highly selective and sensitive NATs and providing insights for designing various practical point-of-care testing (POCT) systems. Herein we report a new type of lateral flow assay (LFA) based on fluorescein-switching, enabled by nucleic acid-templated photooxidation of reduced fluorescein by riboflavin tetraacetate (RFTA). The LFA design leverages the fact that a reduced form of fluorescein, which weakly binds to gold nanoparticle (GNP)-conjugated anti-fluorescein antibodies, is oxidized in the presence of target nucleic acids to yield its native state, which then strongly binds to the antibodies. The study involved designing and optimizing probe sequences to detect miR-6090 and miR-141, which are significant markers for prostate cancer. To minimize background signals of LFAs, sodium borohydride (NaBH4) was specifically introduced as a reducing agent, and detailed procedures were established. The developed LFA system accurately identified low fmol levels of target microRNAs with minimal false positives, all detectable with the naked eye, making the system a promising tool for point-of-care diagnostics.
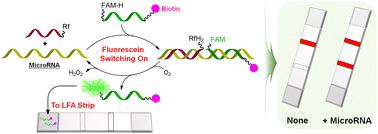
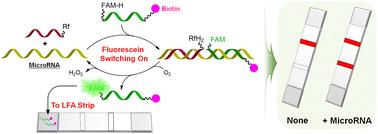
基于荧光素开关的横向流动分析法检测微小核糖核酸
侧流检测法(LFA)是一种经济高效的快速比色技术,可有效地用于医学诊断和生物技术等多个领域的核酸检测(NAT)。鉴于其重要性,开发更多样化、工作机制新颖的 LFA 对于设计高选择性、高灵敏度的 NAT 以及为设计各种实用的床旁检测(POCT)系统提供启示至关重要。在此,我们报告了一种基于荧光素切换的新型侧流检测(LFA),它是由核酸引发的四乙酸核黄素(RFTA)对还原荧光素的光氧化作用促成的。LFA 设计利用了这样一个事实,即与金纳米粒子 (GNP) 结合的抗荧光素抗体结合力较弱的还原型荧光素在靶核酸存在的情况下会被氧化,生成其原生状态,然后与抗体强结合。这项研究涉及设计和优化探针序列,以检测miR-6090和miR-141,它们是前列腺癌的重要标志物。为了尽量减少 LFA 的背景信号,研究人员特别引入了硼氢化钠(NaBH4)作为还原剂,并制定了详细的程序。所开发的 LFA 系统能准确识别低 fmol 水平的目标 microRNA,且假阳性极低,所有目标 microRNA 都能用肉眼检测到。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


