Poison Turned Panacea: Arsenic Trioxide Loaded Hydrogel for Inhibiting Scar Formation in Wound Healing
IF 5.4
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
Without intervention, the natural wound healing process can often result in scarring, which can have detrimental effects on both the physical and mental well-being of patients. Therefore, it is crucial to develop biomaterials that can promote healing without scarring. Regulating the Yes-associated protein-1/PDZ-binding motif (YAP/TAZ) signaling pathway is possible to reduce excessive fibrosis of fibroblasts and proliferation of vascular endothelial cells, ultimately impacting scar formation. Arsenic trioxide (ATO), an ancient drug with medicinal and toxic properties, has shown promise in regulating this pathway. An ATO-loaded hydrogel dressing (ATO@CS/SA) was created to facilitate scarless wound healing, utilizing chitosan (CS) and sodium alginate (SA) to prevent direct contact of ATO with the wound tissue and minimize potential side effects. In vitro studies demonstrated that low concentrations of ATO did not impact cell viability and even promoted proliferation and migration. Co-culturing the hydrogel with fibroblasts and vascular endothelial cells led to decreased expression levels of YAP and TAZ. Animal studies over a 90-day period revealed significant inhibition of scar formation with this system. Histological experiments further confirmed that the decreased expression of YAP and TAZ was responsible for this outcome. In conclusion, when administered at the appropriate dose, ATO can be repurposed from a traditional poison to a therapeutic agent, effectively suppressing excessive cell fibrosis and blood vessel proliferation and offering a novel approach to scar-free treatment.
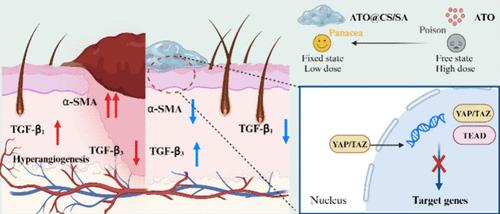
毒药变灵丹:用于抑制伤口愈合中疤痕形成的三氧化二砷负载水凝胶
如果不进行干预,伤口的自然愈合过程往往会留下疤痕,这对患者的身心健康都会产生不利影响。因此,开发能够促进伤口愈合而不留疤痕的生物材料至关重要。调节Yes相关蛋白-1/PDZ结合基团(YAP/TAZ)信号通路可以减少成纤维细胞的过度纤维化和血管内皮细胞的增殖,最终影响疤痕的形成。三氧化二砷(ATO)是一种具有药用和毒性的古老药物,在调节这一通路方面已显示出前景。为了促进无疤痕伤口愈合,我们利用壳聚糖(CS)和海藻酸钠(SA)制成了一种含 ATO 的水凝胶敷料(ATO@CS/SA),以防止 ATO 与伤口组织直接接触,并将潜在的副作用降至最低。体外研究表明,低浓度的 ATO 不会影响细胞活力,甚至还能促进细胞增殖和迁移。将水凝胶与成纤维细胞和血管内皮细胞共同培养,可降低 YAP 和 TAZ 的表达水平。为期 90 天的动物实验表明,该系统能显著抑制疤痕的形成。组织学实验进一步证实,YAP 和 TAZ 的表达减少是造成这一结果的原因。总之,在给药剂量适当的情况下,ATO 可以从传统的毒药转变为治疗药物,有效抑制细胞过度纤维化和血管增生,为无疤痕治疗提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Biomaterials Science & Engineering
Materials Science-Biomaterials
CiteScore
10.30
自引率
3.40%
发文量
413
期刊介绍:
ACS Biomaterials Science & Engineering is the leading journal in the field of biomaterials, serving as an international forum for publishing cutting-edge research and innovative ideas on a broad range of topics:
Applications and Health – implantable tissues and devices, prosthesis, health risks, toxicology
Bio-interactions and Bio-compatibility – material-biology interactions, chemical/morphological/structural communication, mechanobiology, signaling and biological responses, immuno-engineering, calcification, coatings, corrosion and degradation of biomaterials and devices, biophysical regulation of cell functions
Characterization, Synthesis, and Modification – new biomaterials, bioinspired and biomimetic approaches to biomaterials, exploiting structural hierarchy and architectural control, combinatorial strategies for biomaterials discovery, genetic biomaterials design, synthetic biology, new composite systems, bionics, polymer synthesis
Controlled Release and Delivery Systems – biomaterial-based drug and gene delivery, bio-responsive delivery of regulatory molecules, pharmaceutical engineering
Healthcare Advances – clinical translation, regulatory issues, patient safety, emerging trends
Imaging and Diagnostics – imaging agents and probes, theranostics, biosensors, monitoring
Manufacturing and Technology – 3D printing, inks, organ-on-a-chip, bioreactor/perfusion systems, microdevices, BioMEMS, optics and electronics interfaces with biomaterials, systems integration
Modeling and Informatics Tools – scaling methods to guide biomaterial design, predictive algorithms for structure-function, biomechanics, integrating bioinformatics with biomaterials discovery, metabolomics in the context of biomaterials
Tissue Engineering and Regenerative Medicine – basic and applied studies, cell therapies, scaffolds, vascularization, bioartificial organs, transplantation and functionality, cellular agriculture
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


